CELLETS® – Microcrystalline Cellulose Pellets. The safe and easy way to multi-particulate dosage forms
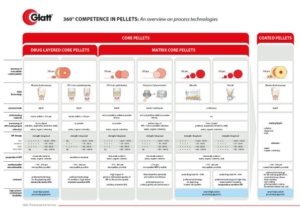
CELLETS® – Microcrystalline Cellulose Pellets – are predestined for multi-particulate dosage forms. Multi-particulates have striking advantages compared to monolithic dosage forms, such as:
- low variability of gastric emptying
- lower dependency on nutrition state
- low risk of localized high drug concentrations in the GI
- reduced risk of sudden dose dumping
- lower intra- and inter-individual variability
- high control on time and place of drug delivery
This means firstly higher safety and secondly better compliance for your patients, and added-value for your products.
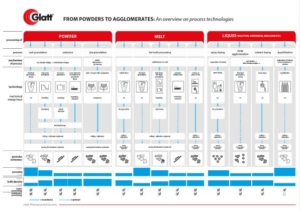
However, historically the way to successful formulation of multiparticulate dosage forms was bumpy. But: CELLETS® with their unique features are now offering streamlined formulation and processing opportunities, making formulator’s life easier, and your patient’s lives safer.