BSC Class I APIs in oral formulations
APIs of BSC Class I in oral formulations represent one of four groups following the Biopharmaceutical Classification System (BCS). The BCS predicts potential effects of oral formulations on the human drug oral bioavailability. Thereby, it is a scientific tool differentiating the drug substances on the basis of solubility and intestinal permeability under prescribed condition. While the solubility classification is based on a United States Pharmacopoeia (USP) aperture, the classification of permeability is based on a comparison to the intravenous injection.
| Permeability | Class I
High solubility High permeability |
Class II
Low solubility High permeability |
| Class III
High solubility Low permeability |
Class IV
Low solubility Low permeability |
|
| Solubility | ||
Table 1: Biopharmaceutics Classification System (BCS).
BSC Class I
BSC Class I drugs are characterized by a high solubility and a high permeability (Table 1). Typical drugs are Amantadine, Diazepam, Itopride HCl, Paracetamol, or Zidovudine [1]. High solubility is given in cases that the highest strength is soluble in pH ranges from pH 1 to pH 6 at body temperature, and a high permeability means an extent of absorption in humans of at least 85%.
Due to high water solubility of BSC class I drugs, a potential substrate in pharmaceutical formulations for oral drugs needs to be water insoluble. In case of MUPS (Multiple Unit Pellet Systems) or capsule dosage forms, pellets of Microcrystalline Cellulose – MCC pellets – are the first choice. Depending on the pellet size, they serve as starter beads in MUPS formulations (smaller than about 800 µm) or in capsules (larger than approx. 800 µm).
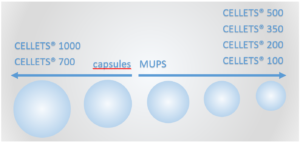
Figure 1: Pellet sizes for potential BSC Class I MUPS and capsule formulations.
MCC spheres or pellets for BSC Class I capsule formulations:
CELLETS® 1000 (1000-1400 µm)
CELLETS® 700 (700-1000 µm)
MCC spheres or pellets for MUPS formulations:
CELLETS® 500 (500-710 µm)
CELLETS® 350 (350-500 µm)
CELLETS® 200 (200-355 µm)
CELLETS® 100 (100-200 µm)
Further information
Are you working with BSC Class IV actives? Read more here.
References
[1] https://www.pharmaspecialists.com/, time: Feb 16th, 2022.

 Ingredient Pharm
Ingredient Pharm