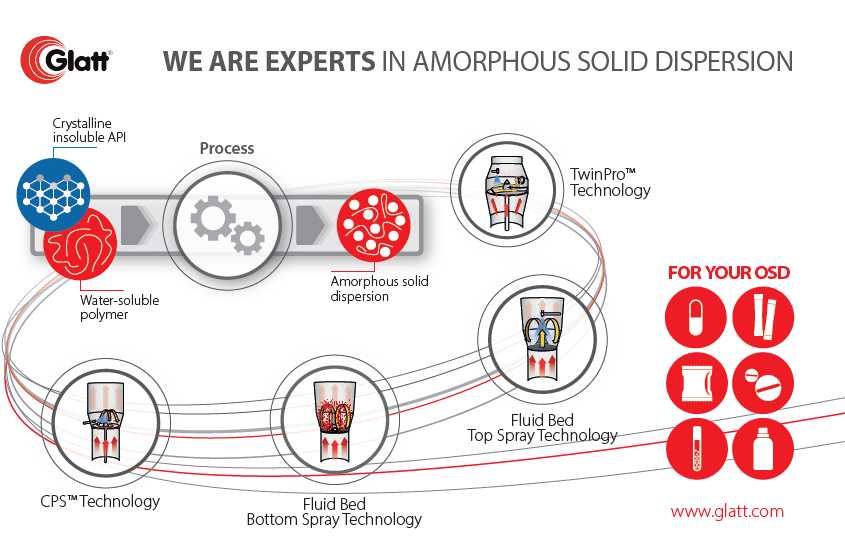
We identified, that amorphous solid dispersions gain in importance as they increase the solubility and dissolution rate of poorly water-soluble drugs. There are severall attempts, in which each of them positive aspects and certain issues occur. It’s time, drawing amorphous solids dispersions in a more general context and sheding some more light on elementary aspects. We like to point on an excellent summary given by Thomas Rades and Keita Kondo [Rades_2022]. Before switching over, we like to emphasis and anticipate one message: The lastest attempts for amorphous solid dispersions is using CELLETS® 175 (MCC spheres) which do not only act as drug carrier, but – due to best friability – as milling balls. You might like follow this attempt with MCC starter beads, so please contact us for getting some materials. Let’s now read more from Rades et al.:
Draw-back on Amorphous solid dispersions
Amorphization is a promising approach to improve the solubility and dissolution rate of poorly water-soluble drugs as amorphous solids lack a crystal lattice with long-range order [1]. However, since amorphous forms are thermodynamically unstable due to a high chemical potential compared to crystalline forms, amorphous drugs exhibit low physical stability and finally recrystallizes [2], [3]. Thus, strategies to stabilize amorphous forms are essential in the development of amorphous products and include the design of amorphous solid dispersions (ASDs) [4], [5] and co-amorphous formulations [6], [7], [8]. ASDs are the most common approach for preparing amorphous products and involve glass formation by molecularly dispersing drug compounds into an amorphous polymer [4], [5]. However, ASD preparations may require a large quantity of polymer to stabilize amorphized drug due to their low miscibility with drug molecules [9], leading to a high bulk volume of the amorphous products. Co-amorphous systems have recently attracted attention as an alternative approach to amorphous formulations and include the formation of a single amorphous phase in which multiple low molecular weight initially crystalline materials (including drug compounds) are uniformly mixed at the molecular levels [6], [7], [8]. Co-amorphous mixtures typically exhibit high physical stability and dissolution characteristics [6], [7], [10].
Co-amorphous systems are typically classified as drug-drug combinations and drug-excipient mixtures. In the former combinations, amorphous phases comprise two drug compounds, which act as a stabilizer for each other by forming intermolecular interactions [11], [12], [13]. These formulations are expected to offer a combination of drugs to enhance the therapeutic effects but their applicability is limited as drug-drug combinations forming co-amorphous solids are not necessarily suitable for combination therapy, or require fixed doses, not necessarily suitable for co-amorphization. In the co-amorphous drug-excipient systems, low molecular-weight substances (including organic acids [14], sugars [15] and amino acids [16]) act as a co-former and their properties and mixing ratio with the drug affect dissolution characteristics and physical stability of the resulting co-amorphous mixtures [8], [10]. Recently, various combinations of drug compounds and amino acids were systematically investigated [17], [18], indicating that co-amorphous mixtures with high dissolution characteristics and physical stability can be produced by selecting amino acids that can form interactions with the target drug compounds (e.g. pairs of acidic drugs and basic amino acids). Therefore, amino acids are a promising co-former class for co-amorphous formulations.
Preparation of co-amorphous mixtures has been reported using melt quenching [13], [19], spray drying [20], [21], and ball milling [16], [22]. Since the resulting solids are in cake or powder forms (regardless of the preparation method), downstream processes including milling and granulation are usually essential to produce final dosage forms such as capsules and tablets for oral administration [23]. These processes can lead to increased risk of phase separations and crystallization due to moisture, thermal, and mechanical stresses. In ASD systems, to avoid the problems due to the downstream processes, one-step preparations of ASD granules by amorphizing drug compounds during the granulation process using fluidized bed processors [24], [25], [26], [27], [28], [29], [30] and high shear granulators [31], [32], [33], [34] have been investigated. However, to our knowledge, there are no reports on one-step preparation methods for co-amorphous granules. In the first part of the current study, we investigated the feasibility of solventless amorphization and pelletization using a high shear granulator and could obtain fully amorphized indomethacin-layered pellets by simply mixing indomethacin crystals and microcrystalline cellulose spheres without using solvent and heating. Indomethacin crystals were pulverized and amorphized due to collisions with the spheres and subsequently are deposited on the surface of the spheres. Therefore, we hypothesized that co-amorphous mixture-layered pellets can be produced through a one-step amorphization and pelletization process using this technique, as the preparation of co-amorphous mixtures has previously been performed by mechanical activation [16], [22]. Furthermore, this technique holds promise as an economical as well as sustainable approach to manufacture co-amorphous formulations as the need for solvent and/or heat is eliminated.
In previous research, various combinations of indomethacin and amino acids for co-amorphous preparations were systematically investigated. The findings suggest that arginine is an excellent co-former for indomethacin to prepare co-amorphous mixtures with fast dissolution characteristics and high physical stability [18], as an amorphous salt is formed due to strong interactions between the acidic drug indomethacin and the basic amino acid arginine [35], [36]. In the current study, to investigate whether co-amorphous layer pellets can be produced through a one-step amorphization and pelletization process, indomethacin and arginine were selected as the model drug and the co-former, respectively. In the first part of this study, indomethacin crystals were mixed with microcrystalline cellulose spheres (with various mean diameters of 140 μm, 195 μm, 275 μm, 414 μm, and 649 μm) at a weight ratio of 1:10 using a high shear granulator [added: TMG1/6, Glatt GmbH, Binzen, Germany]. Fully amorphized indomethacin-layered pellets were obtained using carriers of 414 μm in diameter, whereas partially amorphized indomethacin-layered pellets were obtained using carriers of 195 μm in diameter. This difference was likely due to the higher impact forces of larger carriers promoting mechanical activation of indomethacin crystals. In this part of the study, to clarify the effects of using arginine on the amorphization and pelletization of indomethacin, the smaller cellulose spheres of 195 μm in diameter were employed as carrier particles. Indomethacin crystals and arginine crystals were initially mixed at various molar ratios (1:1, 2:1, and 3:1), and then the resulting mixtures were high shear granulated with microcrystalline cellulose spheres at a weight ratio of 1:10. The resulting composite particles were characterized using solid-state and particle analytical techniques. To identify effective co-amorphization approaches, we examined high shear mixing under various jacket temperatures. Furthermore, physical stability and dissolution characteristics of co-amorphous layer pellets were investigated.
References
[Rades_2022] K. Kondo, T. Rades, 181 (2022) 183-194. doi:10.1016/j.ejpb.2022.11.011
[1] B.C. Hancock, M. Parks, Pharm. Res. 17 (2000) 397-404.
[2] L. Yu, Adv. Drug Deliv. Rev. 48 (2001) 27-42.
[3] L.R. Hilden, K.R. Morris, J. Pharm. Sci. 93 (2004) 3-12.
[4] T. Vasconcelos, S. Marques, J. das Neves, B. Sarmento, Adv. Drug Deliv. Rev. 100 (2016) 85-101.
[5] S. Baghel, H. Cathcart, N.J. O’Reilly, J. Pharm. Sci. 105 (2016) 2527-2544.
[6] R. Laitinen, K. Lobmann, C.J. Strachan, H. Grohganz, T. Rades, Int. J. Pharm. 453 (2013) 65-79.
[7] R.B. Chavan, R. Thipparaboina, D. Kumar, N.R. Shastri, Int. J. Pharm. 515 (2016) 403-415.
[8] S.J. Dengale, H. Grohganz, T. Rades, K. Lobmann, Adv. Drug Deliv. Rev. 100 (2016) 116-125.
[9] S. Janssens, G. Van den Mooter, J. Pharm. Pharmacol. 61 (2009) 1571-1586.
[10] R. Laitinen, K. Lobmann, H. Grohganz, P. Priemel, C.J. Strachan, T. Rades, Int. J. Pharm. 532 (2017) 1-12.
[11] S. Yamamura, H. Gotoh, Y. Sakamoto, Y. Momose, Eur. J. Pharm. Biopharm. 49 (2000) 259-265.
[12] M. Allesø, N. Chieng, S. Rehder, J. Rantanen, T. Rades, J. Aaltonen, J. Control. Release 136 (2009) 45-53.
[13] K. Lobmann, R. Laitinen, H. Grohganz, K.C. Gordon, C. Strachan, T. Rades, Mol. Pharm. 8 (2011) 1919-1928.
[14] Q. Lu, G. Zografi, Pharm. Res. 15 (1998) 1202-1206.
[15] M. Descamps, J.F. Willart, E. Dudognon, V. Caron, J. Pharm. Sci. 96 (2007) 1398-1407.
[16] K. Lobmann, H. Grohganz, R. Laitinen, C. Strachan, T. Rades, Eur. J. Pharm. Biopharm. 85 (2013) 873-881.
[17] G. Kasten, H. Grohganz, T. Rades, K. Lobmann, Eur. J. Pharm. Sci. 95 (2016) 28-35.
[18] G. Kasten, K. Lobmann, H. Grohganz, T. Rades, Int. J. Pharm. 557 (2019) 366-373.
[19] A. Teja, P.B. Musmade, A.B. Khade, S.J. Dengale, Eur. J. Pharm. Sci. 78 (2015) 234-244.
[20] A. Beyer, L. Radi, H. Grohganz, K. Lobmann, T. Rades, C.S. Leopold, Eur. J. Pharm. Biopharm. 104 (2016) 72-81.
[21] E. Lenz, K.T. Jensen, L.I. Blaabjerg, K. Knop, H. Grohganz, K. Lobmann, T. Rades,
- Kleinebudde, Eur. J. Pharm. Biopharm. 96 (2015) 44-52.
[22] K.T. Jensen, F.H. Larsen, C. Cornett, K. Lobmann, H. Grohganz, T. Rades, Mol. Pharm. 12 (2015) 2484-2492.
[23] B. Demuth, Z.K. Nagy, A. Balogh, T. Vigh, G. Marosi, G. Verreck, I. Van Assche, M.E. Brewster, Int. J. Pharm. 486 (2015) 268-286.
[24] D.B. Beten, K. Amighi, A.J. Möes, Pharm. Res. 12 (1995) 1269-1272.
[25] H.-O. Ho, H.-L. Su, T. Tsai, M.-T. Sheu, Int. J. Pharm. 139 (1996) 223-229.
[26] N. Sun, X. Wei, B. Wu, J. Chen, Y. Lu, W. Wu, Powder Technol. 182 (2008) 72-80.
[27] A. Dereymaker, D.J. Scurr, E.D. Steer, C.J. Roberts, G. Van den Mooter, Mol. Pharm. 14 (2017) 959-973.
[28] A. Dereymaker, J. Pelgrims, F. Engelen, P. Adriaensens, G. Van den Mooter, Mol. Pharm. 14 (2017) 974-983.
[29] T. Oshima, R. Sonoda, M. Ohkuma, H. Sunada, Chem. Pharm. Bull. 55 (2007) 1557-1562.
[30] H.J. Kwon, E.J. Heo, Y.H. Kim, S. Kim, Y.H. Hwang, J.M. Byun, S.H. Cheon, S.Y. Park, D.Y. Kim, K.H. Cho, H.J. Maeng, D.J. Jang, Pharmaceutics 11(3) (2019) 136.
[31] N.S. Trasi, S. Bhujbal, Q.T. Zhou, L.S. Taylor, Int. J. Pharm. X 1 (2019) 100035.
[32] A. Seo, P. Holm, H.G. Kristensen, T. Schæfer, Int. J. Pharm. 259 (2003) 161-171.
[33] T. Vilhelmsen, H. Eliasen, T. Schaefer, Int. J. Pharm. 303 (2005) 132-142.
[34] Y.C. Chen, H.O. Ho, J.D. Chiou, M.T. Sheu, Int. J. Pharm. 473 (2014) 458-468.
[35] K.T. Jensen, L.I. Blaabjerg, E. Lenz, A. Bohr, H. Grohganz, P. Kleinebudde, T. Rades, K. Lobmann, J. Pharm. Pharmacol. 68 (2016) 615-624.
[36] K.T. Jensen, F.H. Larsen, K. Lobmann, T. Rades, H. Grohganz, Eur. J. Pharm. Biopharm. 107 (2016) 32-39.
More information on ASD
Read more about amorphous solid dispersions in our application notes.

 ingredient pharm
ingredient pharm