Abstract
This application note is based on content from Pohlen et al. [1]. Simvastatin (CAS number 79902-63-9) is a cholesterol-lowering agent with a low bioavailability of 5% [2,3]. This API is formulated as a lipid based drug delivery system for oral uptake. Two technologies, which are spray drying and fluidized bed layering technologies were compared with respect to the process and product characteristics of otherwise similar Simvastatin loaded dry emulsion systems. Investigated parameters are the process yield, encapsulation efficiency, relative product stability, particle morphology, drug content, and the relative increase in bioavailability.
Enhancing bioavailability
Some of the recently discovered new chemical entities (NCE) show a low solubility and high permeability (BCS class II), or even low permeability in the case of very high lipophilicity (BCS class IV).
| Material |
Company |
| Simvastatin |
Krka, SI |
| 1-oleoyl-rac-glycerol,
Magnesium stearate,
Tween® 20 |
Merck, D |
| Pharmacoat 603 |
ShinEtsu, JAP |
| Miglyol® 812 |
Sasol, D |
| Pearlitol SD 200 |
Roquette, F |
| CELLETS® 200 |
HARKE Pharma, D |
| Avicel® PH 101,
Lactose mesh 200 |
Lek, d.d., SI |
Table 1: Used Material and origin.
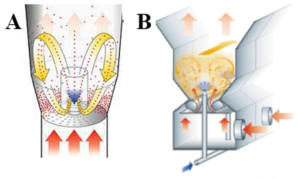
This means a major challenge for formulation development in terms of assuring drug bioavailability [4,5]. A strategy for increasing the solubility are lipid based drug delivery systems (LBDDS). As main advantage, they are likely to solubilize the API and make it available for the absorption into the bloodstream [6]. Additionally, converting the liquid or semi-solid LBDDS into solid dosage forms eliminates undesired characteristics such as a lack of chemical stability and product portability, susceptibility for drug recrystallization and costly manufacturing [7]. Furthermore, solid dosage form solutions allow benefits, such as easy powder processing, flow and compression behavior, controlled drug release, improved patient safety. Among others, dry emulsions are a type of solidified LBDDS and allow carrying and releasing the encapsulated lipophilic API. In the following, some solidification process technologies are introduced. The required parameter for Wurster fluidized bed and spray drying are displayed in Table 2 and Table 3, respectively.
Opposite to the spray drying process, the fluidized bed process employs CELLETS® 200 as starter beads for layering. Several formulations are composed by Pohlen et al., the materials are listed in Table 1.
| Parameter |
Value |
| Setup |
Glatt Fluidized bed Dryer Model GPCG-1 (Glatt, D) |
| Two-fluid
Schlick nozzle |
0.8 mm |
| cap opening diameter |
2.50 mm |
| Inlet airflow rate |
130 m3/h |
| Inlet air temperature |
47 °C to 56 °C |
| outlet air / product temperature |
34 °C |
| spraying rate |
5 g/min to 9 g/min |
| atomizing air pressure |
2 bar |
| Gap to Wurster insert bottom edge |
17.5 mm |
| Drying time |
180 s @ 42 °C |
| Starter pellets |
200 g |
| starting
emulsion |
1000 g |
Table 2: Parameters and values for Fluidized bed layering.
| Parameter |
Value |
| Setup |
Mini Spray Dryer B-290 (Büchi, CH) |
| Two-fluid
nozzle |
1.4 mm |
| cap opening diameter |
2.20 mm |
| Inlet airflow rate |
28 m3/h |
| Inlet air temperature |
145°C to 175 °C |
| outlet air / product temperature |
75 °C to 80 °C |
| spraying rate |
6 g/min |
| Drying time |
180 s @ 80 °C |
| Starter pellets |
200 g |
| starting
emulsion |
1000 g |
Table 3: Parameters and values for spray drying.
Process yield
Spray drying results on average in lower process yield than the fluidized bed results. The process yield for spray drying experiments is in average value of 71.5 %, and of 83.3 % for fluidized bed layering experiments. It is assumed, that in spray drying process adhesion of the smallest particles to the cyclone walls or outtake through the air stream occur.
Drug content
An averaged API content at 9.34 mg/g in fluidized bed experiments, and at 22.2 mg/g for spray dried dry emulsions is reached. Although spray drying offers a much higher drug content and more flexible formulations, the content variation between replicates is increased. The use a swirl air generator in the fluidized bed equipment increases process stability and allows an even larger amount of oil to be incorporated. It is possible increase the maximum amount of API to 22 mg/g onto the starter pellets. Anyhow, the fluidized bed technology suffers from sticky effects of oil phases which is not a big deal in spray drying processes.
Encapsulation efficiency
A low encapsulation efficiency shall be avoided as it causes drug losses during processing and increased production costs. The encapsulation efficiency in fluidized bed experiments is at 80.0 %, compared to spray drying experiments being at 68.4 %. A main issue of the spray drying technology might be higher process temperature leading to a higher risk of API degradation. Spray drying also suffers from a larger surface-to-volume area which might induce an increased risk of oxidation during the drying process.
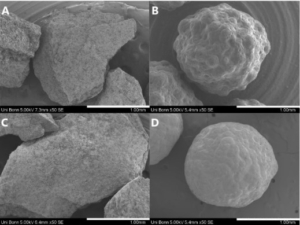
Morphology and particle size
The main advantage of fluidized bed technology is the use of starter pellets, which are perfectly spherical starter beads. Following, API coating results in highly spherical coated particles with a high level of monodispersity and an average particle size around 336 µm (D50 value). Not mentionable, that spray drying technology results in smaller average particle sizes at 56 µm (D50 value), but the morphology shows a coarse, rough and undefined surface. In turn, dry emulsion layered pellets have better flow properties [8].
Redispersibility and oil droplet size
All re-dispersed oil droplets have a size of a few micrometers between less than 1 µm and less than 7 µm. Fluidized bed layering technology generally leads to larger droplets. Considering also the probable bimodal nature of the droplet size distribution, fluidized bed layering provides a narrower size distribution and thus better results. In turn, the fluidized bed technology might provide slightly better bioavailability.
Product stability
Stability is measured by means of the one-month relative drug content stability. The particles produced in the fluidized bed technology show a better one-month relative drug content stability than particles produced by spray drying. This might be caused by the higher monodispersity, larger particles and smoother surfaces. All properties minimize the risk of API gradation, treatment failure, or toxicity.
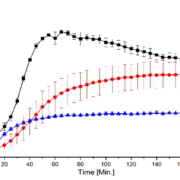
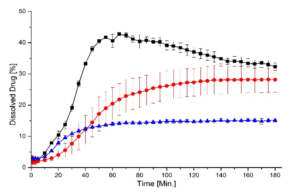
Dissolution
Both technologies show a superior dissolution behavior compared to the dissolution of pure crystalline API (less than 3 %) or a generic API tablet (less than 10 %). It has to be stated, that both technologies allow dissolution rates of more than 80 % within the first 30 minutes, wherein the Spray drying products show a slightly better and faster dissolution rate.
Bioavailability
Bioavailability of formulations from fluidized bed layered dry emulsion pellets provide the highest increase in relative bioavailability within the examined formulations, confirming that fluidized bed technology is superior to spray drying technology for potent or low dose APIs.
Summary
Fluidized bed layering and spray drying technology have been selected for analyzing the properties of dry emulsions. Simvastatin was selected as API, encapsulated in the dry emulsion.
Fluidized bed layering technology uses starter cores, such as CELLETS® as a dry emulsion carrier system, while spray drying does not.
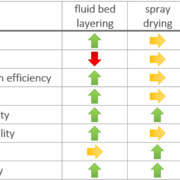
The main advantage of the fluidized bed technology is the higher process yield, the better encapsulation efficiency and redispersibility, the defined morphology of the product causing better process handling and product stability.
Spray drying technology allows a higher drug content with better chances of formulation variation, and an even faster and more complete dissolution (Figure 1).
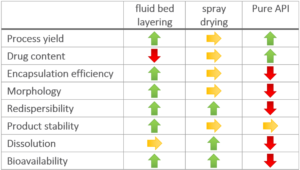
Figure 1: Advantages of technological methods compared to a pure API usage.
References
[1] M. Pohlen, J. Aguiar Zdovc, J. Trontelj, J. Mravljak, M. G. Matjaž, I. Grabnar, T. Snoj and R. Dreu, Eur J Pharm Biopharm (2021), S0939-6411(21)00353-2, doi:10.1016/j.ejpb.2021.12.004
[2] S. Geboers, J. Stappaerts, J. Tack, P. Annaert and P. Augustijns, Int. J. Pharm. 510 (2016) 296-303, doi:10.1016/j.ijpharm.2016.06.048
[3] T. Taupitz, J.B. Dressman and S. Klein, Eur J Pharm Biopharm. 84 (2013) 208-218, doi:10.1016/j.ejpb.2012.11.027.
[4] T. Das, C.H. Mehta and U.Y. Nayak, Drug Discov. Today 25(7) (2020) 1206-1212, doi:10.1016/j.drudis.2020.04.016
[5] G.L. Amidon, H. Lennernäs, V.P. Shah and J.R. Crison, Pharm. Res. 12 (1995) 413-420, doi:10.1023/a:1016212804288.
[6] H. Mu, R. Holm and A. Müllertz, Int. J. Pharm. 453 (2013) 215-224, doi:10.1016/j.ijpharm.2013.03.054.
[7] P. Joyce, T.J. Dening, T.R. Meola, H.B. Schultz, R. Holm, N. Thomas and C.A. Prestidge, Adv. Drug Deliv. (2018), doi:10.1016/j.addr.2018.11.006.
[8] X. Fu, D. Huck, L. Makein, B. Armstrong, U. Willen and T. Freeman, Particuology. 10 (2012) 203-208, doi:10.1016/j.partic.2011.11.003


 Ingredient Pharm
Ingredient Pharm