Abstract
This case study on Atomoxetine HCl pellets is a short abstract of the publication by Y.D. Priya et al. [1].
Atomoxetine is a medication used to treat attention deficit hyperactivity disorder (ADHD) [2]. The API is marketed under the trade names Atomoxetine, Atomoxe, Agakalin, and Strattera (initially launched) [3]. Atomoxetine is an extremely bitter API. As being initially launched for children as capsules or tablets, the paediatric compliance by improved taste-masking and the simplified administration to paediatrics are in focus of this study.
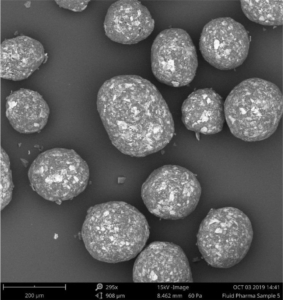
A multi-unit particulate pellet coating (MUPS) was selected as oral dosage form. The fluidized bed technology (with Wurster column) was employed for coating and layering processes. This is a well-known technology, which Is for instance offered by Glatt. Starter cores were coated with the API, followed by layering with a polymeric coating for which realized the taste-masking.
Atomoxetine layering
Starter cores are made of Microcrystalline Cellulose (MCC) in sizes comparable to CELLETS® 200, while a fair efficiency of drug layering was observed with the combination of HPMC (Hydroxypropyl methyl cellulose) and HPC (Hydroxypropyl cellulose) as binders. The composition of API layering is presented in Table 1. The drug dispersion was sprayed onto the MCC pellets with an inlet temperature between 50 °C and 55 °C and a fluidized bed temperature between 35 °C and 40 °C.
| API layering material | Composition |
| Starter core | |
| MCC pellets | 58.00 |
| API layering | |
| Atomoxetine HCl | 25.00 |
| Hydroxypropyl methylcellulose | 3.50 |
| Hydroxypropyl Cellulose | 3.50 |
| Low-Substituted Hydroxypropyl Cellulose | 5.00 |
| Talc | 5.00 |
| Purified Water | Qs |
| Total weight (mg) | 100.00 |
Table 1: Formulation of API layered pellets.
Taste-masking coating
The polymeric taste-masking layer is made of a methacrylate co-polymer (Eudragit EPO) providing an excellent coating with taste masking properties for fine particles and tablets. The composition of the taste-masking suspension is shown in Table 2. The inlet temperature is between 40 °C and 45 °C, and fluidized bed temperature is between 25 °C and 30 °C.
| Polymeric coating material | Composition |
| Drug Layered pellets | 100.00 |
| Eudragit EPO | 25.00 |
| Sodium Lauryl Sulfate | 2.500 |
| Stearic acid | 3.750 |
| Talc | 6.25 |
| FD&C Yellow No. 6 | 0.50 |
| FD&C Red No. 3 | 0.05 |
| Purified Water | Qs |
| Total weight (mg) | 138.050 |
Table 2: Formulation of polymeric coating suspension.
The efficiency of taste-masking was benchmarked by a bitterness rating on human volunteers. Figure 1 shows, that the taste sensitivity identifies a bitterness at 6 µg/ml API concentration and an extreme bitterness at 7 µg/ml API and higher concentration. Thus, the threshold bitterness of Atomoxetine HCl is 6 µg/ml.
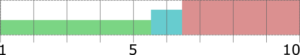
Figure 1: Concentration of drug solution (µg/ ml). Bitter intensity ratings from no bitterness (green), bitterness (blue), extremely bitter (red).
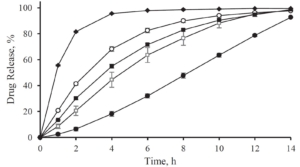
All the volunteers felt bitter taste when the drug layered pellets were coated with 6.25 mg of Eudragit EPO. Whereas in the pellets coated with 12.5 mg and 18.75 mg of Eudragit EPO, bitter taste was masked up to 15 seconds after keeping the tablet in the mouth, and later all the human volunteers felt bitter taste. When the concentration of Eudragit EPO was increased to 25 mg, the bitter taste of Atomoxetine HCl was completely taste-masked and no volunteer was felt bitter taste.

Figure 2: In-Vivo Taste evaluation in healthy human volunteers.
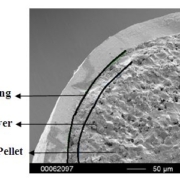
Figure 3 depicts the entire particle size of a taste-masked MCC pellet coated with the Atomoxetine drug layer and 25 mg of Eudragit EPO. The average particle size of the taste-masked pellets is between 180 µm and 250 µm, assuming, that no gritty feeling of particles in patient’s mouth will appear. It should be said, that a micronization of Atomoxetine HCl was deemed to be necessary for the drug layering process. Micronization minimized the surface roughness of the API layered pellet so that an efficient taste-masking coating can be applied.
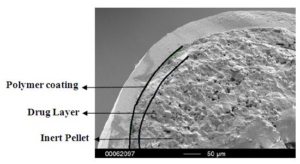
Figure 3: SEM picture of cross section of a Taste masked pellets coated with 25 mg Eudragit EPO.
Summary
MCC pellets in the size of about 200 µm were layered with Atomoxetine. HPMC and HPC were used as binders, realizing a precise surface definition for a subsequent taste-masking coating. The taste-masking was most efficient at a polymeric concentration of 25 mg. Keeping the size of the coated pellets below 300 µm avoids a gritty feeling and thus increase the patient’s compliance.
This study by Priya et al. indicated that the fluidized bed process produced the most appropriate taste masked pellets of Atomoxetine HCl for oral disintegrating tablets.
References
[1] Y.D. Priya et al., Int J Pharm Pharm Sci, (6) 7, (2014) 110-115
[2] “Atomoxetine Hydrochloride Monograph for Professionals”. Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 4 April 2019. Retrieved 22 March 2019.
[3] ROTE LISTE 2017, Verlag Rote Liste Service GmbH, Frankfurt am Main, ISBN 978-3-946057-10-9, (2017) 162.