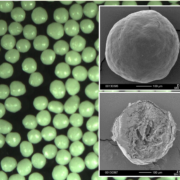
short for: Microcrystalline Cellulose.
See also: Microcrystalline Cellulose.
Posts
Abstract
Theophylline is a powerful active used for the acute treatment of respiratory distress. Its bioavailability and uptake rates are high. Drug carrier systems are pellets made of sugar or microcrystalline cellulose (MCC). This case study will point on the specific advantages of MCC pellets.
Layering on starter pellets
Basically, theophylline is an alkaloid that occurs in nature together with other purine alkaloids such as caffeine and theobromine, but it occurs in comparably small fractions up to 0.25 %. Anyhow, it can be synthetically composed. In application, theophylline is used for the acute treatment of respiratory distress due to airway constriction in bronchial asthma and other obstructive airway diseases.
After oral administration theophylline is rapidly and completely absorbed in the gastrointestinal tract (GIT). Retard preparations are used for long-term treatment, reaching their maximum effect after around six to eight hours [1].
Typical carrier systems are sugar and MCC pellets (Cellets®). By subsequent layering, retard and individual release profiles can be achieved. For both types of starter pellets, a drug solution for 200 g pellets (batch size) was formulated in the following way as listed in Table 1.
| Parameter | weighted mass |
| theophylline | 8.32 g |
| PVP K30 | 0.67 g |
| distilled water | 80.0 g |
| ammonia 25 % | 4.0 g |
Table 1: Substances for a drug solution for 200 g batch size.
Process Technology
The formulation results in a drug load of 4.2 %. A Wurster tube at 0.8 cm was used with a processing temperature at 50 °C. In contrast to MCC pellets, sugar pellets are soluble in water. Therefore, process parameters are slightly different, since the process for sugar spheres requires a slower start to avoid sticky particles (Table 2). Obviously, the slower process start required for the sugar pellets results in an additional time consumption of +50 % compared to the Cellets® process.
| Parameter | Sugar pellets | Cellets® |
| Batch size | 200.0 g | |
| Wurster tube | 0.8 cm | |
| Fluid bed temperature | 50 °C | |
| Inlet air volume (pressure) | 0.4 bar | 0.35 bar |
| Atomizing air pressure | 2.3 bar | 1.8 bar |
| Spray rate | 0.41 g/min | 0.73 g/min |
| Process time | 218 min | 145 min |
| Drying period | 30 min | |
Table 2: Process parameter for the formulation with sugar pellets and Cellets®.
Finalized pellets
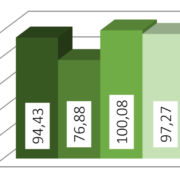
The processed drug layered pellets show a size distribution as shown in Figure 1. Here, the variation between the batches of the sugar pellets are more pronounced (18.6 %) than for the batches of Cellets® (2.8 %).
The formulation results in a drug load of 4.2 %. A Wurster tube at 0.8 cm was used with a processing temperature at 50 °C. In contrast to MCC pellets, sugar pellets are soluble in water. Therefore, process parameters are slightly different, since the process for sugar spheres requires a slower start to avoid sticky particles (Table 2). Obviously, the slower process start required for the sugar pellets results in an additional time consumption of +50 % compared to the Cellets® process.
| Parameter | Sugar pellets | Cellets® |
| Batch size | 200.0 g | |
| Wurster tube | 0.8 cm | |
| Fluid bed temperature | 50 °C | |
| Inlet air volume (pressure) | 0.4 bar | 0.35 bar |
| Atomizing air pressure | 2.3 bar | 1.8 bar |
| Spray rate | 0.41 g/min | 0.73 g/min |
| Process time | 218 min | 145 min |
| Drying period | 30 min | |
Table 2: Process parameter for the formulation with sugar pellets and Cellets®.
The processed drug layered pellets show a size distribution as shown in Figure 1. Here, the variation between the batches of the sugar pellets are more pronounced (18.6 %) than for the batches of Cellets® (2.8 %).
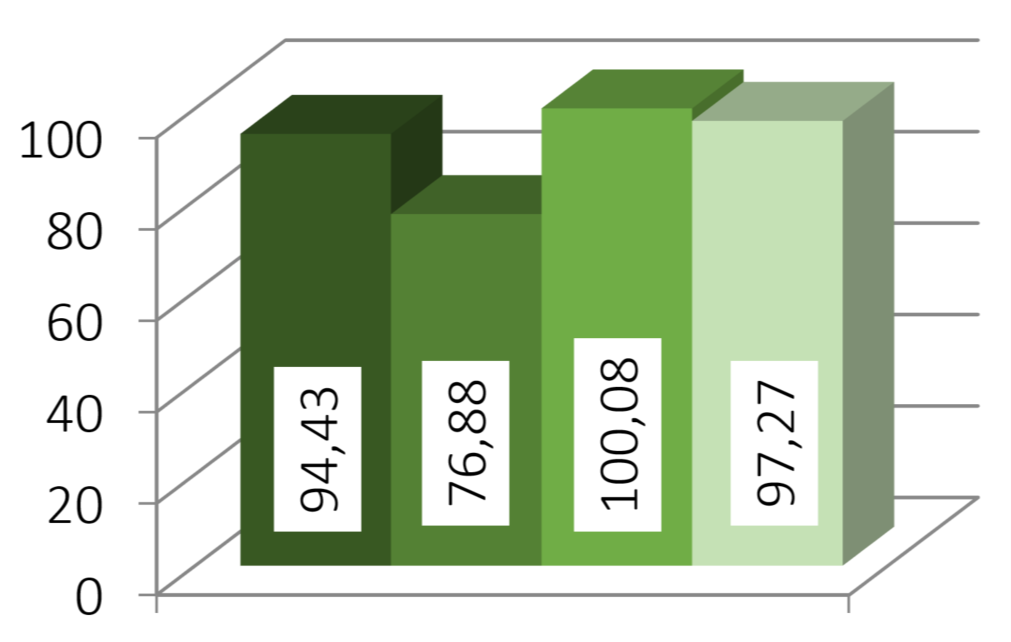
Theophylline size distribution
Figure 1: Analysis of batches. From left to right: (1) best batch sugar pellets, (2) worst batch sugar pellets, (3) best batch Cellets®, (4) worst batch Cellets®.
Summary
In this case study, the coating of pellets with theophylline was investigated. A targeted drug load of 4.2 % was reached. By sophisticated formulation, further improvements towards optimized release profiles of the active in the GIT can be performed. Here, MCC pellets are superior to sugar pellets in terms of reproducibility, process time and quality rating after coating.
Acknowledgement
We acknowledge Dr. Riedel (Bayer) for assisting this case study.