Abstract
Coating uniformity is a critical parameter in coating processes in novel pharmaceutical formulations. Speaking about pellet technology, coating and layering are the main methods for implementing drug functionalities, such as modified release of the active, taste-masking properties and further more. Coating uniformity guaranties not only upholding functionalities of the formulation, but also prevent risks such as dose dumping.
This application note is based on a publication of Wörthmann et al. [1] and focuses on selected aspects which are related to starter cores.

Figure 1: Microscopic image of Cellets® 1000, magnification 100x.
Materials and techniques
Coating was applied on highly spherical starter cores Cellets® 1000 (Figure 1). The pellets have a relatively narrow size distribution with a mean particle size of d50 = 1197 μm, a standard deviation of σ = 113 μm, and particle density of 1.4 g/cm3. For analyzing the coating uniformity, stearin (54 % stearic acid and palmitic acid) and hydrogenated palm oil were used. For the hot-melt coating experiments a lab-scale Wurster fluidized bed was used. The overspray rate was estimated to 8 % (w/w). Processed particles were analyzed by image analysis (Figure 2) and micro-computed-tomography (μCT) (Figure 3). 2D and 3D software analysis were further conducted for the evaluation of the sphere dimension, layer thickness and coating uniformity.
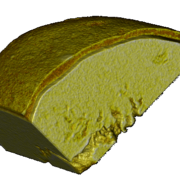
Figure 2 shows a wax-coated particle, where the coating thickness varies and delamination is clearly visible (Figure 3). Small pores and fractions of the coating layer area are obvious.
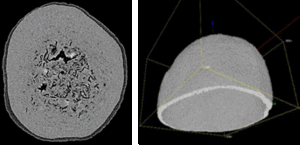
Figure 2: Images of coated pellets are used for a stepwise evaluation of the particle shell thickness. A: original image; B: segmented coating layer. Further software calculation steps are not shown here.
These undesired artefacts result from imperfect parameters, such as spreading mechanism, temperature fluctuations, viscosity, or drop size.
The coating layer thickness is analyzed for three particles of the same batch (Figure 4) using 5 % (w/w) stearin at a spraying rate of 1 g/min. The layer thickness varies between approximately 2 µm to 30 µm. A mean coating thickness is found between 12 µm and 16 µm.
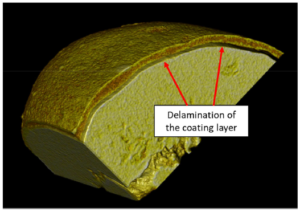
Figure 3: Portion of a micro-computed-tomography image of a wax-coated particle showing.
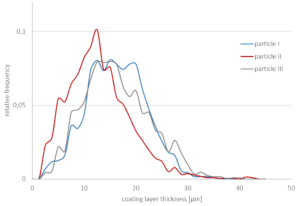
Figure 4: Relative frequency of the coating layer-thickness of three particle shells from the same batch using 5 % (w/w) stearin at a spraying rate of 1 g/min. Mean thicknesses: particle I (blue): 15.5 μm, particle II (red): 12.4 μm, and particle III (grey): 15.6 μm.
In terms of customer safety and of compliance aspects, not only statistical information about the layer thickness are of interest. In case of inhomogeneous layers, taste-masking functionalities or even uncontrolled dose dumping might occur. In this context, a single-particle analysis is mandatory. 3D µCT is a powerful tool, which is complementary to existing methods, such as laser imaging methods, 2D analysis or thickness estimations. The analyzed mean thickness deviates by approximately 13 % among these methods (Figure 5).
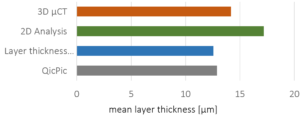
Figure 5: Mean layer-thicknesses measured using different methods. Relative standard deviation: 13 %.
Summary
Microcrystalline cellulose pellets (Cellets®) are used to study coating uniformity. 3D μCT can be a powerful tool to assess the quality of the final product coating and facilitates the selection of an appropriate combination of core particles and coating material. 3D visualization methods allow a critical single-particle analysis with a resolution of up to 2 µm. Furthermore, the determination of the particle’s uncoated surface area can be specified.
Acknowledgement
Prof. Heiko Briesen, Mario Wörthmann (Technical University Munich) and team are gratefully acknowledged for serving content for this note.
Research was financially supported by the Ministry of Economics and Energy (BMWi) and FEI (Germany) via project AiF 19970 N. Equipment funded by Deutsche Forschungsgemeinschaft (DFG, Germany) 198187031.
References
[1] B.M. Woerthmann, J.A. Lindner, T. Kovacevic, P. Pergam, F. Schmid, H. Briesen, Powder Technology 378 (2021) 51–59

 glatt.com
glatt.com