Posts
Abstract
Amorphous solid dispersions layered pellets solve a problem of poorly water soluble drugs. Speaking about oral drug formulations, drug carrier solutions based on starter cores are suitable for several drug classes and open new opportunities for modified drug release profiles. Layering and coating techniques, such as Wurster fluid bed process at different batch sizes, are well established.
However, an increasing number of poorly water soluble drugs challenges modern formulations. A novel approach improving the solubility of those drugs is to formulate them as amorphous solid dispersions (ASD) with a suitable polymer candidate [1]. In this study, Nifedipine was used as a model drug. Nifedipine manages angina, high blood pressure, Raynaud’s phenomenon, and premature labor [2].
Formulation & techniques
ASD formulations can be performed by hot-melt extrusion or spray drying technique. Both techniques have disadvantages such that hot-melt extrusion cannot be employed for temperature-sensitive drugs [3], and spray drying needs a further compaction step not to result in fine powder with poor flowability, broad particle size distribution and high sensitivity to electrostatic charge. Therefore, a further compaction step is required to obtain a freely flowable product [4].
In this context, two techniques for the preparation of ASDs are compared: A 6”-Wurster fluid bed with Type-C bottom plate (Glatt, Germany) and spouted bed (ProCell5™ with Zig-Zag-sifter, Glatt, Germany) are used.
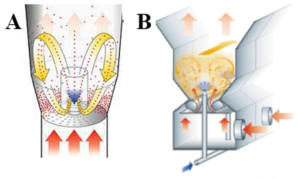
Fig. 1: A: 6”-Wurster fluid bed; B: ProCell5™ spouted bed.
The formulation contains the drug and a stabilizing co-polymer (Kollidon®, KVA64, BASF, Germany). Nifedipine and Kollidon are mixed resulting in a drug load of 40 % (w/w) and dissolved in Acetone (30 % w/w solid content).
| Parameter | FB | SB |
| Spray rate [g/min] | 20 | 20-35 |
| Product temp. [°C] | 50-60 | 50-60 |
| Process gas temp. [°C] | 65 | 80 |
| Process air flow [m³/h] | 180-200 | 65-120 |
| Spraying nozzle diameter [mm] | 1.2 | 1.2 |
| Spraying pressure [bar] | 2.0 | 0.5 |
Table 1: Manufacturing parameters for fluid bed (FB) and spouted bed (SB).
In the fluid bed process, microcrystalline pellets (Cellets® 500, IPC Dresden, Germany) were layered with the spraying solution such that a drug load of 21.8 % (w/w) is reached. In the spouted bed process, fine powder is generated by spray drying, further agglomeration and layering. An overview on the process parameters is given in Table 1.
Dissolution Tests
Dissolution tests were conducted in a PBS buffer at pH 6.8 and 37 °C (± 0.5 °C). A physical mixture of Nifedipine and KVA64 (40 % w/w drug load) is used as reference.
Results
In the following, results from both experiments, which are amorphous solid dispersions layered pellets (fluid bed) and ASD pellets from direct pelletization (spouted bed) are compared.
Flowability and particle size
ASD layered pellets show a better sphericity, higher level of monodispersity and better flowability properties than the ASD pellets from direct pelletization (Figure 2). Nonetheless, it has to be pointed out that both techniques result in a high particle quality for capsule filling. Analysis data is shown in Table 2.
| Parameter | FB | SB |
| D10 [µm] | 824 ± 23 | 559 ± 28 |
| D50 [µm] | 943 ± 13 | 732 ± 50 |
| D90 [µm] | 1091 ± 11 | 1374 ± 410 |
| Bulk density [g/L] | 427 | 280 |
| Flowability [s/100g] | 12.1 | 16.2 |
Table 2: Analysis of ASD layered pellets (FB) and ASD pellets from direct compaction (SB).

Fig. 2a: SEM images of processed pellets. A: ASD layered pellets based on Cellets® (FB)

Fig. 2b: SEM images of processed pellets. B: ASD pellets from direct pelletization (SB)
Dissolution profiles
Independent from the processing technique, pellets achieved an approximately factor 2 higher end concentration than the physical mixture. Pellets obtained from the fluid bed process showed a clear supersaturation phase after 1 hour and a generally higher dissolution rate than pellets obtained from the spouted bed process. Contrarily, the dissolution rate of the latter pellets approaches the supersaturation phase more continuously after 3 hours.
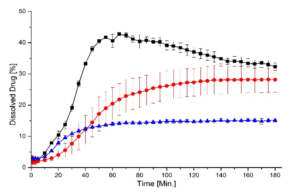
Fig. 3: Dissolution as a function of time. Black: ASD layered pellets (FB). Red: ASD pellets from direct pelletization (SB). Blue: physical mixture.
Summary
Both techniques, fluid bed and spouted bed as well, can be employed for manufacturing amorphous solid dispersions with good flow properties and dissolution profiles. Both techniques can be scaled up to pilot and production scale for batch or continuous manufacture of freely flowable ASDs. Cellets® serve stable and reliable cores for this venture.
Acknowledgement
We gratefully acknowledge Dr. Annette Grave and Dr. Norbert Pöllinger (Glatt Pharmaceutical Services, Germany), and Prof. Karl G. Wagner and Marius Neuwirth (University Bonn, Germany).
References
[1] T. Vasconcelos, B. Sarmento, and P. Costa, Drug Discovery Today, 12(23): 1068-1075 (2007)
[2] “Nifedipine”. The American Society of Health-System Pharmacists. Retrieved: Sept 17, 2019.
[3] J. Breitenbach, European Journal of Pharmaceutics and Biopharmaceutics, (54)2: 107-117 (2002)
[4] I. Weuts et al., Journal of Pharmaceutical Sciences, (100)1: 260-274 (2011)
Abstract
Microcrystalline cellulose pellets (MCC) and sugar are well-known materials in pellet technology. Pellet technology describes the drug load onto starter pellets for controlled release formulations by Wurster process or others. Inert pellets are made of microcrystalline cellulose, while water soluble pellets are composed of sugar. Both material classes show desirable characteristics, such as a narrow particle size distribution, sphericity, surface smoothness. Also the batch-to-batch reproducibility and robustness of starter cores is high. A comparison does not seem to be that easy …
Starter cores in the micron range
Respecting the final application, the initial size of starter pellets defines the final size of the drug loaded pellet. In case of several layers of API and excipients, the initial size is factorized by the layering process. Pellet sizes in a range from 200 µm to 700 µm are frequently used (Table 1). We will focus on three size classes within this range and compare MCC pellets with those made of sugar.

Figure 1: MCC pellets (here: Cellets® 200) are shown with good sphericity and striking surface smoothness.
Small-sized pellets starting at 200 µm
Small-sized pellets with sizes starting at 200 µm and larger, exhibit a comparably large surface-to-volume ratio. This can be beneficial in some applications. For example, taste-masking of bitter API is accessible.

Figure 2: Sugar pellets (here: 50/70 mesh) are shown with moderate sphericity and reduced surface smoothness.
Figure 1 displays a microscopic image of MCC Cellets® 200 and Figure 2 displays the image of sugar pellets in 50/70 mesh, respectively. It is obvious, that for small-sized pellets, the sphericity and surface smoothness of MCC pellets is superior.
| Size | MCC | Sugar |
| small | Cellets® 200 | 50/70 mesh |
| Medium | Cellets® 350 | 40/50 mesh |
| large | Cellets® 500 | 25/30 mesh |
Table 1: Size definition of MCC and sugar pellets.
Mid-sized pellets up to 500 µm
This class of pellets is frequently used for multi-layer coating technologies. Easy processing and reliable batch-to-batch control are positive aspects. Exemplary application is a hydrocortisone formulation for peadiatrics. Again, Figure 3 (MCC pellets) and Figure 4 (sugar pellets) show advantages in surface properties for the MCC material.
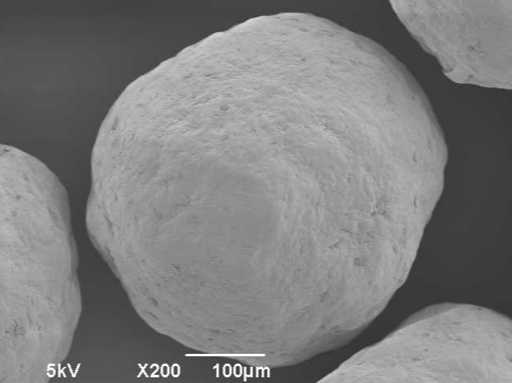
Figure 3: MCC pellets (Cellets® 350) are shown.

Figure 4: Sugar pellets (40/50 mesh) are shown
Large-sized pellets above 500 µm
In some applications, larger pellet sizes are requested. Let’s have short excurse into straws which can contain larger pellets in dry state. Upon use by sucking liquid through the straw, the API coating dissolves immediately while the pellet remains in the straw by simple filters.
In this size range the striking advantages of MCC pellets are not of immediate importance, but still visible.

Figure 5: MCC pellet above 500 µm (Cellets® 500).

Figure 6: Sugar pellet above 500 µm (25/30 mesh).
Summary
Microcrystalline cellulose pellets (Cellets®) show superior surface and sphericity properties compared to sugar pellets. In case of non-dissolving applications, MCC pellets are first choice. As sugar pellets exhibit strong dissolution in water, there is still a fair application range for them.
Abstract
Microcrystalline Cellulose (MCC) pellets represent a chemically inert class of active pharmaceutical ingredients (API) carriers. A narrow particle size distribution (PSD) maximizes control over content uniformity. In this case study, we will focus on measuring the particle size distribution and on sphericity.
Pellets for oral drug forms
MCC pellets are used as starter beads for API loading. Low or high drug dose loading is technically feasible. These pellets are made of pure MCC and provide a robust platform for delivery of one or multiple APIs. Certain processing technologies for pellet coating allow these starter beads to be compatible with soluble or insoluble APIs, e.g. by Wurster bottom spray [1,2] or Rotor dry powder layering technology [3]. Coated pellets can be filled into capsules, or compacted into multiple-unit pellet system (MUPS) tablets [4], where a tight PSD maximizes control over content uniformity.
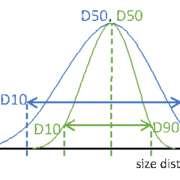
Particle size distribution maximizes the control over content uniformity in applications of complex oral dosing forms. Speaking about uniform or monodisperse particles, these information always point to general information of the particular system, not of the individual particle itself. Therefore, PSD is a globular measure allowing simple, easy and fast analysis of the particulate matter. Major key information from a PSD measure are the so called D-values. A Dx value represents a dimension, where a ratio of X particles is smaller. For reasons of simplicity weighted functions, such as number, radius or volume, are not. Extending these metrics to D10 and D90 additionally informs about the width of the entire size distribution (Figure 1).
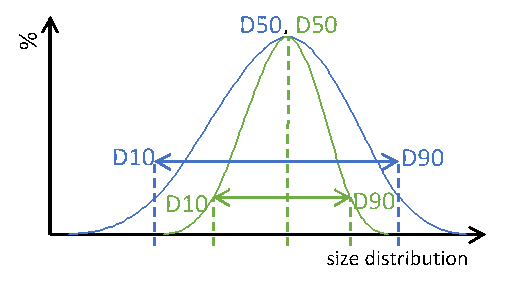
Particle size distribution of two different particle systems with identical median dimension D50. Blue: wide PSD, green: narrow PSD. Dotted lines are guides to the eyes.
Figure 1: Particle size distribution of two different particle systems with identical median dimension D50. Blue: wide PSD, green: narrow PSD. Dotted lines are guides to the eyes.
Dimensions of pellets
In this study, imaging technology (Horiba, Camsizer) was employed for the size analysis. Representatively, more than 50 charges of Cellets® 100 and Cellets® 500 (Figures 2-3) have been analyzed for the D10, D50 and D90 values.
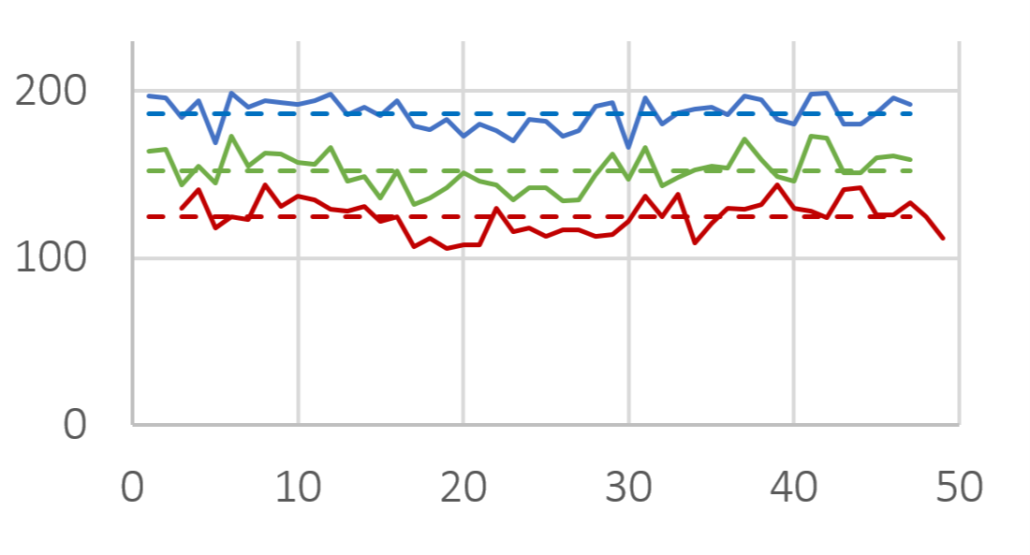
D10 (red), D50 (green) and D90 (blue) value for several Cellets® 100 charges. Solid lines are measures, dashed lines represent the averaged value of all charges. The standard deviation is below 10 %.
Figure 2: D10 (red), D50 (green) and D90 (blue) value for several Cellets® 100 charges. Solid lines are measures, dashed lines represent the averaged value of all charges. The standard deviation is below 10 %.
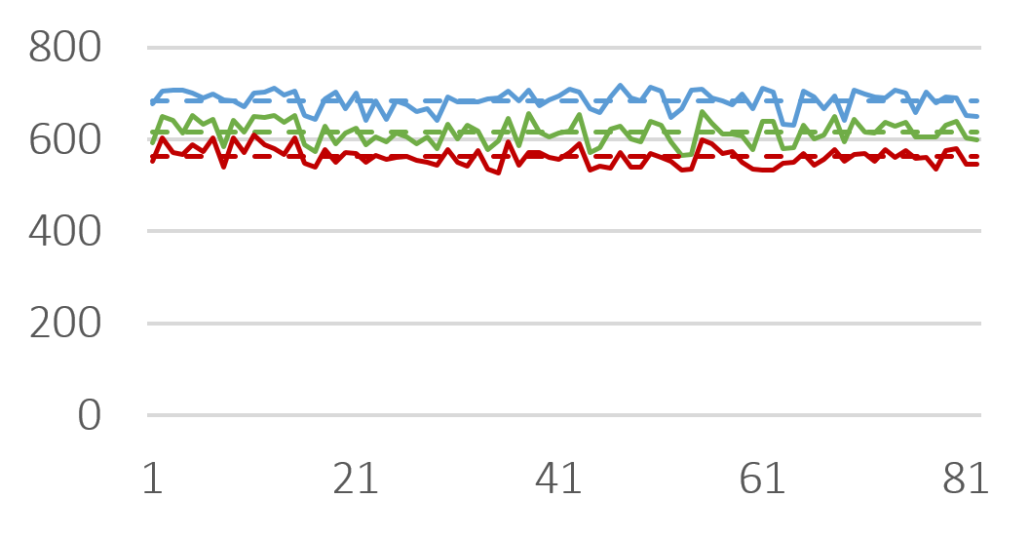
D10 (red), D50 (green) and D90 (blue) value for several Cellets® 500 charges. Solid lines are measures, dashed lines represent the averaged value of all charges. The standard deviation is below 4 %.
Figure 3: D10 (red), D50 (green) and D90 (blue) value for several Cellets® 500 charges. Solid lines are measures, dashed lines represent the averaged value of all charges. The standard deviation is below 4 %.
The results show only slight variations in the PSD between the charges. The standard deviation is smaller than 4 % (Cellets® 500) and smaller than 10 % (Cellets® 100) which confirms a high reproducibility in production (Table 1). Both values are remarkably good for technical spheres. Furthermore, none of the charges was out of specifications and fit into the desired size distribution between 500 µm and 710 µm easily. The close gap between D10 and D90 clearly identify an excellent monodispersity.
| Standard deviation | Cellets 100 | Cellets 500 |
| of D10 | 8.28 % | 3.97 % |
| of D50 | 7.12 % | 3.52 % |
| of D90 | 4.68 % | 3.11 % |
Table 1: Standard deviation for D10, D50 and D90 values Cellets® 100 and Cellets® 500 charges.
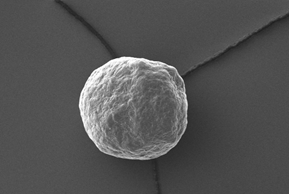
Electron microscopy yield perfect imaging data of the MCC pellets’ surfaces. Magnification: 250x, working distance 8.0 mm, voltage: 10 keV.
Figure 4: Electron microscopy yield perfect imaging data of the MCC pellets’ surfaces. Magnification: 250x, working distance 8.0 mm, voltage: 10 keV.
Perfect sphericity? – Yes!
For a more detailed shape analysis, electron microscopy yield perfect imaging data of the MCC pellets’ surfaces (Figure 4). Additionally, MCC pellets have a distinguishing friability.
Summary
Microcrystalline Cellulose (MCC) pellets show excellent chemically inertness, high degree of sphericity, narrow size distribution and high reproducibility in production. These properties make Cellets® becoming one of the first choice for inert API carriers. We have proven these excellent properties for Cellets® 100 and Cellets® 500. The obtained results are representative for other size classes ranging from 100 µm to 1400 µm.
Acknowledgement
We acknowledge IPC Process-Center (Dresden, Germany) for the analytics, and Fraunhofer IFAM (Dresden, Germany) for recording the electron microscopic pictures.
References
[1] H. R. Norouzi, International Journal of Pharmaceutics, Volume 590 (2020) 119931
[2] D. Jones, Developing Solid Oral Dosage Forms, Pharmaceutical Theory And Practice (2009) 807-825
[4] S. Abdul, A. Chandewar, S. Jaiswal, Journal of Controlled Release, Volume 147(1) (2010) 2-16
Abstract
Starter beads such as pellets made of microcrystalline cellulose (MCC) are frequently used in the formulation of oral drug delivery systems, e.g. multiparticulates [1] or multi-unit pellet system (MUPS) tablets [2]. Certain properties are requested to MCC pellets. We shed some light on sphericity size and friability in this note.
Starter beads for MUPS tablets
MUPS tablets consist of pellets which are compressed – assisted by excipients such as disintegrants and fillers. The pellets used are usually functional coated to achieve desired drug release profiles.
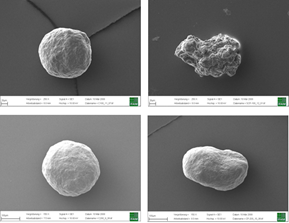
Top: Inert Cellets® 100 (100-200 µm, left) in comparison with another MCC sphere (75-212 µm, right). Bottom: Inert Cellets® 200 (200-350 µm, left) in comparison with another MCC sphere (150-300 µm).
Figure 1: Top: Inert Cellets® 100 (100-200 µm, left) in comparison with another MCC sphere (75-212 µm, right). Bottom: Inert Cellets® 200 (200-350 µm, left) in comparison with another MCC sphere (150-300 µm).
The characteristics of the starter bead as a neutral carrier should therefore include high sphericity (Figure 1), constant particle size distribution and smooth surface. These aspects count especially for the formulation of low dosed highly active APIs.
For the application in MUPS tablets small size and high mechanical stability (low friability) are of interest to achieve desired drug loading and avoid film damage during compression.
Size
Any question relating to optimized drug load and coating layers of pellets is a question of size and sphericity of the starter beads.
So, what is the main influence of size? Size needs to be considered for achieving desired drug load in relation to a total dimension of the pellet. While the total dimension of the pellet is mainly defined by the application – e.g. processing as a capsule, tablet or sachet –, the initial pellet size defines the maximum thickness of coating levels (Figure 2). Size might also be a matter of content uniformity with low dosed API and also needs to be mentioned by means of processability, which is in particular electrostatic loading or sticking. Particle size distribution influences the dissolution profile.

Figure 2: Sketch of a functionally coated pellet. The size of the initial pellet (green) defines the maximum thickness of all coating layers (blue) which may contain API and excipients, as well.
Figure 2: Sketch of a functionally coated pellet. The size of the initial pellet (green) defines the maximum thickness of all coating layers (blue) which may contain API and excipients, as well.
Sphericity
Sphericity is a strong parameter which influence depends on drug loading and coating levels. Also for the control of dissolution profile where specific surface area and content uniformity play important roles, the influence of sphericity needs to be understood (Figure 3). Please do not forget, that with decreasing sphericity, the flow probabilities of powders are decreasing (powder rheology), which might affect process properties such as powder transport.

Figure 3: Sketch of non-spherical starter beads (green) with coating layers (blue). Coating layer thickness and dissolution profiles are hard to control in this case.
Figure 3: Sketch of non-spherical starter beads (green) with coating layers (blue). Coating layer thickness and dissolution profiles are hard to control in this case.
Thus, starter beads of uniform size (distribution) and sphericity are the better solution for overcoming these issues by simplifying drug formulation and processing. Such starter beads can be pellets of MCC, sugar or tartaric acid. MCC pellets surely show perfect initial conditions as they exhibit chemical inertness and therefore can be combined with several APIs. In case of weakly basic APIs, tartaric acid pellets are advantageous.

Figure 4: A pellet inside a compressed MUPS tablet. The starter bead is surrounded by a coating layer of exemplarily excipient or API. A powdery excipients matrix surrounds the coated pellet. Friability is absolutely low.
Figure 4: A pellet inside a compressed MUPS tablet. The starter bead is surrounded by a coating layer of exemplarily excipient or API. A powdery excipients matrix surrounds the coated pellet. Friability is absolutely low.
Figure 4 shows a cross-section of a pellet in the matrix of a compressed MUPS tablet. It is mentionable, that due to low friability a high degree of sphericity as well as surface smoothness are kept after compression and film damage of coating layers is not identified.
Summary
Cellets® offer a perfect combination of chemical inertness towards the selection of the API and physical properties that allow optimized and stable processing in a fluid bed process for layering and coating of the starter beads. Main advantages are the low friability, smooth surface, sphericity and narrow size distributions.
Cellets® starter beads therefore provide excellent conditions for controlled drug dissolution profiles.
Acknowledgement
We acknowledge Fraunhofer IFAM (Dresden, Germany) for providing electron microscopic images.
References
[1] Pöllinger N, Drug Product Development for Older Adults—Multiparticulate Formulations. In: Stegemann S. (eds) Developing Drug Products in an Aging Society. AAPS Advances in the Pharmaceutical Sciences Series, vol 26 (2016). Springer, Cham. https://doi.org/10.1007/978-3-319-43099-7_16
[2] Bhad ME, Abdul S, Jaiswal SB, Chandewar AV, Jain JM, Sakarkar DM. MUPS tablets—a brief review. Int J Pharm Tech Res. 2010;2:847–55

 ingredientpharm
ingredientpharm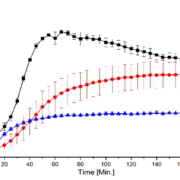
 ingredientpharm
ingredientpharm