Critical aspects of starter spheres in oral pellet formulations one should consider
Starter spheres in oral pellet formulations are of increasing importance in modern pharmaceutical drug dosage forms. Among others, capsules, multiparticulates or sachet solutions gain in diversifying drug administration routes and targeting multiple patient groups from newborn (pediatrics) to seniors (geriatric medicines). A common aspect of these formulation attempts are starter spheres. These spheres form the initial base of each formulation. By conducting coating and layering technologies, API dissolution, drug release time, taste-masking properties and corporate brand requirements are pushed to optimum. Thus, it sounds reasonable to introduce most critical aspects or parameters of these spheres. In the following, we like to share information on the particle size distribution, the friability and sphericity, as well.
Critical aspect no. 1: Particle size distribution
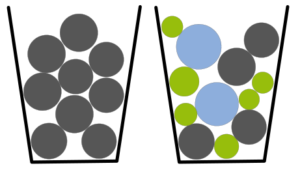
The particle size distribution is for sure the most relevant quality parameter of starter beads in respective oral drug formulations. Changes in the particle size distribution are crucial for the release profile as they directly relate to a variation of surface-per-volume area distribution. In detail, an widening in size distribution induces a non-linear widening of the surface-per-volume area distribution. Thus, particle size distribution attributes the release control and dissolution characteristics of the formulation.
Thereby, the starter bead can be generally be of an pelletized excipient, such as Microcrystalline Cellulose (CELLETS®), Tartaric Acid (TAP®), Sugar or Mannitol. But also granulated excipients by extrusion or matrix API beads as well, have to be seen critically from this point of view.
Experimentally, changes in particle size distribution induces changes in packaging density such that with increasing size distribution, packaging density increases. In turn, also the in-process flow behavior might vary, and an active process control is mandatory in batch-to-batch production.
Critical aspect no. 2: Friability
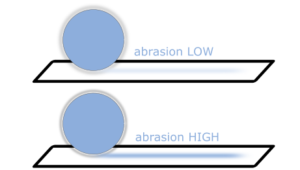
Friability experimentally describes the mechanical resistance of a starter sphere against mechanic friction. It describes the relative amount of material loss on time-limited external mechanical force on the sphere. In friability tests the material’s susceptibility to attrition is evaluated. Therefore, high friability of the naked starter sphere is a performant property for safe coating and layering process from the beginning. Thus, it becomes a remarkable parameter tailoring the opportunities for each formulation. [1]
Critical aspect no. 3: Sphericity
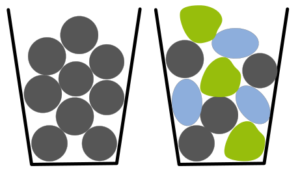
As one of the most interesting parameters for describing surface properties of a starter sphere is sphericity. Sphericity informs about macroscopic aspects of the sphere’s surface, while microscopically the surface smoothness or roughness can be the factor of choice. Sphericity take the value equal to 1 for a perfect sphere symmetry and decays for any changes in surface curvature. Thus, a loss in sphericity attributes an increase in surface per volume ratio. Thus, the effect of losing this quality parameter has similar influence on the pharmaceutical formulation, as seen for the particle size distribution. On the other hand, sphericity has an impact on packaging and flow behavior, where analogous conclusions to friability effects are obvious. Technically, a decrease in sphericity induces a change and decrease in coating layer thickness if you do not react on this by modifying the coating volumes. Experimentally, dissolution and release time are affected negatively.
Critical aspects – individual solutions
By far, this application note is not a sword of Damocles on pharmaceutical pellet formulations. Their advantages clearly outweigh the disadvantages and modern API request for these novel formulations. Anyhow, you should consider some simple tricks to develop formulation recipes which consider these aspects of starter beads in such way, that the formulation exhibits a certain robustness against disruptions.
References
[1] J. Werther, Jens Reppenhagen, Fluidization, Solids Handling, and Processing, 1999

 ingredientpharm
ingredientpharm