Abstract
Modified drug release formulations for suspensions are a perfect solution for children and patients with swallowing difficulties. In many cases, these formulations are based on pellets serving as starter beads. In this report, an attempt on microparticle coating by Mohylyuk et al. [1] is described. Herein, small scaled microcrystalline cellulose pellets (Cellets® 90 and Cellets® 100, Table 1) in the size range smaller than 150 µm are used. Through a modified Wurster fluidized bed process, a yield of 99 % was reached.
| Starter materials | PSD (> 85 %) |
| Cellets® 90 | 63-125 μm |
| Cellets® 100 | 100-200 µm |
Table 1: Size distribution of Cellets® as starter beads in this formulation.
Goals and Formulation
The goal is to investigate a revolutionary platform for sustained-release microencapsulation using the industrial fluidized bed coating technology. Significant challenges of particle cohesion in the process shall be avoided by applying a small quantity of dry powder glidant periodically during the coating process. A highly water-soluble drug, which is metoprolol succinate, is reproducibly microencapsulated on pellet technologies with total pellet sizes of less than 200 µm and a drug release time of 20 hours.
Excipients for extended release profiles
For obtaining a sustained release profile, polymethacrylate-based copolymers, Eudragit RS/RL® 30 D and Eudragit® NM 30 D, were used in combination with a range of anti-tacking agents. The coating onto placebo Cellets® 100 starter beads was performed in a fluidized bed coater with a Wurster insert (Mini-Glatt, Glatt GmbH, Germany) in order to analyze the release profile. Process parameters are shown in Table 2. A small quantity of dry powder glidant was periodically added during processing, so that particle cohesion was eliminated. The optimized excipient composition for the desired release profile is achieved by testing 10 different compositions.
| Parameter | Value |
| Inlet air temperature | |
| Eudragit RS/RL® 30 D | 35-40 °C |
| Eudragit® NM 30 D | 30-35 °C |
| Product temperature | |
| Eudragit RS/RL® 30 D | 25-30 °C |
| Eudragit® NM 30 D | 18-20 °C |
| air flow rate | 18 m3/h |
| Atomization pressure | 1.5 bar |
| Spray rate | 1.1-2.4 g/min |
Table 2: Process parameter for a fluidized bed coater with a Wurster insert. A sustained release drug layer is coated onto placebo Cellets® 100 starter beads.
Drug coating
For drug coating, Cellets® 90 were layered with a suspension of metoprolol succinate in a composition as shown in Table 3.
| Material | Concentration (w/w) |
| Metoprolol succinate | 22.8 % |
| Hypromellose | 0.6 % |
| talc (Pharma M) | 4.0 % |
| Deionized water | 72.6 % |
Table 3: Composition of metoprolol succinate suspension for drug layering onto Cellets® 90.
The metoprolol succinate-loaded Cellets® 90 microparticles were successfully coated with the Eudragit® NM 30 D based aqueous dispersion, achieving a high product yield of 99 % and a final particle size of less than 200 µm (D50 value).
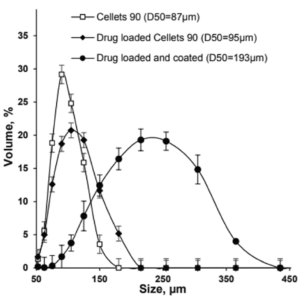
Figure 1: Size distribution of Cellets® 90 as uncoated (empty squares), drug loaded (filled diamonds) and drug loaded and coated (filled circles) particles.
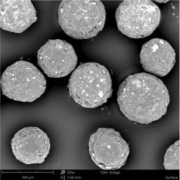
The API loaded and coated starter beads are of high sphericity and show a homogeneous and narrow size distribution, which is shown as a SEM (scanning electron microscope) image in Figure 2.
In dissolution tests, an extended release time of up to 20 hours is obtained and can still be varied by the composition of excipients (Figure 3).
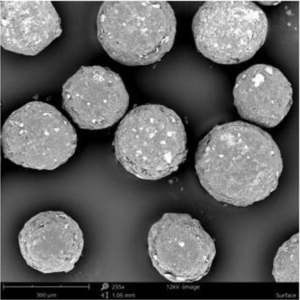
Figure 2: SEM image of drug loaded and coated starter beads. Microparticles show a high level of homogeneity in size distribution.
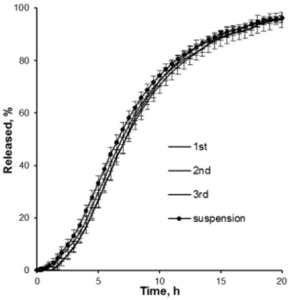
Figure 3: Drug release profiles of three batches of metoprolol succinate loaded and coated Cellets. An extended release of 20 hours is obtained.
Summary
This case study is a short abstract of the publication on microparticle coating by Mohylyuk et al. [1], highlighting the proof of concept for reproducible microencapsulation of a highly water-soluble drug by applying a small quantity of dry powder glidant periodically during Wurster fluidized bed coating. The challenge of particle cohesion in the “down flow” zone was eliminated and a high product yields up to 99% was achieved.
Coated microparticles are in size of less than 200 μm and show a 20 hours sustained drug release profile. These conditions allow the usage in liquid suspensions. Furthermore, the applied technology is scalable. In conclusion, this displays a sustained-release dosage solution, which is suitable for paediatrics and geriatrics with swallowing difficulties.
Acknowledgement
Dr. Fang Liu and her team are gratefully acknowledged for serving content and data for this note.
Fluid Pharma Ltd
Contact: Dr. Fang LIU
College Lane, Hatfield, AL10 9AB, UK
Tel: +44 1707 28 4273
+44 796 3230 628