Abstract
Multiparticulates made of pellets are ideal dosage forms to be used in pediatrics. Having the suitability of paediatric consumers in mind, formulations of small-sized pellets offer a valuable base for increased compliance and improved age-appropriately dosage form. Due to their round shape of pellets, smooth surface area and narrow particle size distribution they can easily be functionally coated [1] to achieve e. g. a taste masking, enteric protection or the controlled release of the active pharmaceutical ingredient (API) in defined parts of the gastro-intestinal (GI) tract. The release profile then often depends on the coating weight gain (thickness) and composition of the functional coating.
Coating weight gain, manufacture and analysis of pellets
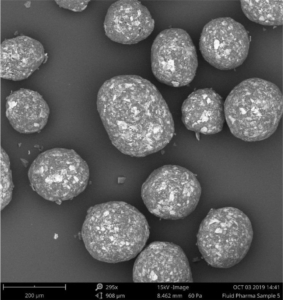
A well soluble drug was used as model API. In a first approach, pellets were produced applying the ProCell technology, a direct pelletization process allowing the production of highly drug loaded matrix pellets (here 95%) in a spouted bed. Two types of pellets were produced: A) with a poly amino saccharide-based binder, followed by a cellulose based seal coating and B) with a polyacrylic acid-based binder, followed by a pH-depending coating. In a second approach the API was layered onto inert starter cores (MCC, CELLETS® 200) by the aid of a cellulose based binder and antitacking agent applying the Wurster technology targeting a drug load of 50 %, followed by a pH-depending coating (C). All three pellets-based populations were functionally coated by a pH-independent sustained release polymer. Samples were taken at pre-defined coating levels for dissolution testing. For API layering and coating a GPCG 1.1 with a 6” Wurster insert was used. Direct pelletization was performed in a ProCell 5. Particle size distribution (PSD) analysis was performed by Eyecon2TM. The particle size is given as numeric or volumetric distribution (e.g. Dn50 or Dv50). The specific surface area is calculated by measuring the true density by gas pycnometry and the Sauter diameter by Laser diffraction. Dissolution was measured in the acid stage (0.1 M HCl), in buffer pH 5.5 and in buffer pH 7.2 over 300 min. The API should not be released in the first 180 min. Between 210 min and 240 min an increased drug release is expected. The dissolution rates at 225 min were compared for the coating levels at 10, 15 and 20 %.
Results
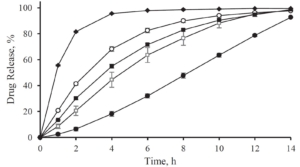
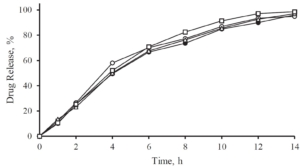
With increasing coating weight gains decreasing dissolution rates at 225 min were measured for the sustained release coating with a good linearity. Matrix PEL (A) show higher dissolution rates comparing the same coating levels than Matrix PEL (B), Wurster pellets showed the strongest decrease with increasing CWG, table 1, figure 1. This correlation was not observed for pH-depending coating (data not shown).
| Dv 50 [µm] | Dn 50 [µm] | PSD mean [µm] | Specific surface area [m2/g] | ||
| A | Matrix PEL | 496 | 475 | 481 | 0,00980 |
| B | Matrix PEL | 461 | 427 | 425 | 0,01210 |
| C | Wurster PEL | 414 | 396 | 401 | 0,01100 |
Table 1. PSD data and specific surface area of starter beads before functional coating.
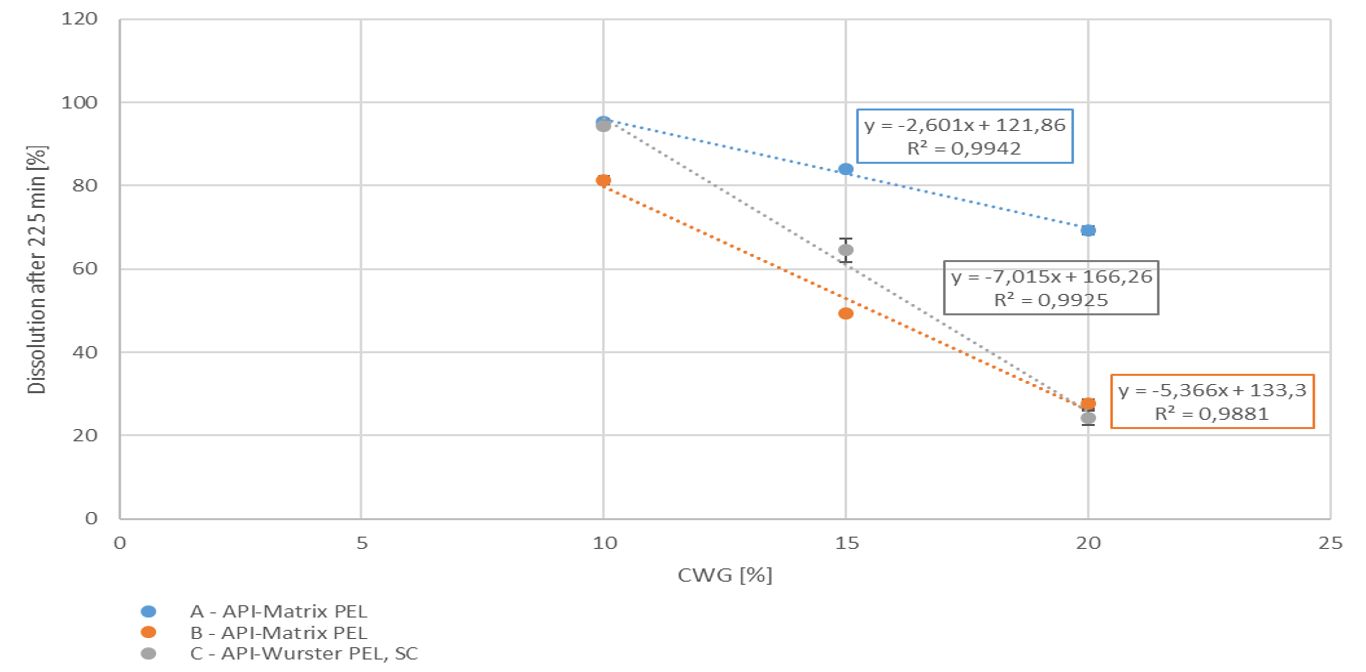
Figure 1. Dissolution at 225 min vs. coating weight gain (CWG)
Summary
Drug loaded pellets were prepared either as matrix pellets applying the ProCell technology, or by layering of starter cores applying the Wurster technology. Both populations were coated with different coating levels of a sustained release functional coating, resulting in decreasing dissolution rates with increasing coating weight gain. Due to the good correlation between coating weight gain and dissolution profile a prediction of the dissolution rate might be possible for pre-defined coating levels. These findings are a crucial step towards novel paediatric formulations with improved dissolution profiles and dosage safety.
References
[1] Palugan, L.; Cerea, M.; Zema, L.; Gazzaniga, A.; Maroni, A. Coated pellets for oral colon delivery, Journal of Drug Delivery Science and Technology 25, 1 – 15 (2015).
This study was presented on 14th annual EuPFI conference, Rome, Italy.

 ingredientpharm
ingredientpharm