Hydrocortisone for paediatrics
Abstract
Hydrocortisone is found on the EMA unmet medical need priority list (2011) for the use in paediatric population for children suffering from all forms of adrenal insufficiency. Although there is a particular need in neonates and in infants, i.e. children younger than two years, there is a main issue. No licensed hydrocortisone preparation for paediatric use is available in Europe. Within the TAIN program (treatment of adrenal insufficiency in neonates and infants) supported by the European Commission [1], oral drug dosage research has been initiated.
By looking at the information we have about the side effects of commonly used drugs, let alone more niche ones, on pediatrics and comparing it with the available data of adults, I assume that pediatric medicine is a field of so many unturned stones. particularly, providing exclusive excipients to formulate pediatric drugs and designing special formulations for this population.
Issues on Hydrocortisone
Hydrocortisone can be given orally, topically, or by injection [2]. After oral administration, free cortisol passes easily through cellular membranes which explains its 100% bioavailability [3]. Paediatrics key characteristics are taste masking (Figure 1) and the requirement of dedicated dosing units. Also the immediate drug release shell be comparable to crushed Hydrocortisone tablets.

Taste masking increases customer compliance of bitter actives in paediatrics.
Transforming these requirements into a development strategy, following focus points need to be considered:
- Dosage change by multiparticulates
- Layering/coating of starter beads demands narrow size distribution and defined smooth surfaces.
These considerations exclude working on standard dry powder forms like rough granules, but recommend the usage of pellets. We will focus on pellets made of microcrystalline cellulose (MCC), which are CELLETS® 350. The main advantage of these MCC pellets are narrow size distribution, smooth surface and chemical inertness. By spray coating, functional layers are coated onto the MCC pellet (Figure 2). Different drug loads can be achieved.
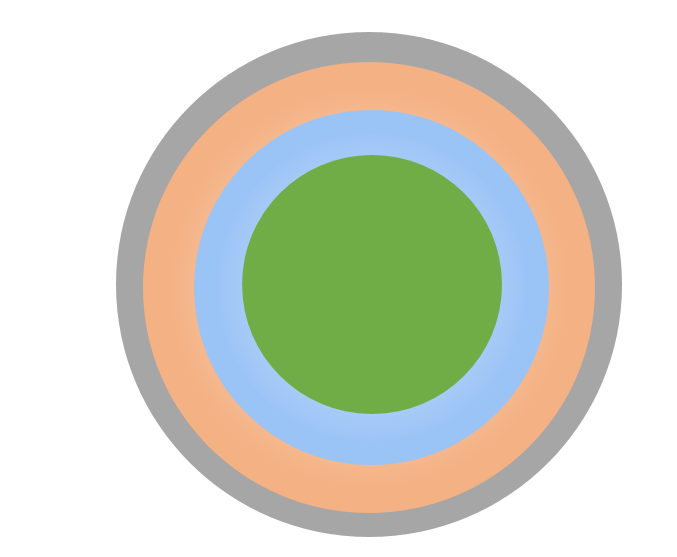
Coated MCC pellet (green). Functional layers: hydrocortisone (blue), seal coat (orange), taste masking (grey).
A concept and selection of feasible excipients provide CELLETS® as starter beads, cellulose derivates as binder, seal coating and taste masking.
The size distribution of CELLETS® 350 is known to be highly narrow (Figure 3). Also critical parameters, such as sphericity and smoothness of the surface are almost perfect which is presented by an electron microscopy image in Figure 4. In turn, a subsequent precise layering of the active and adjustment of optimal time release profiles is possible.
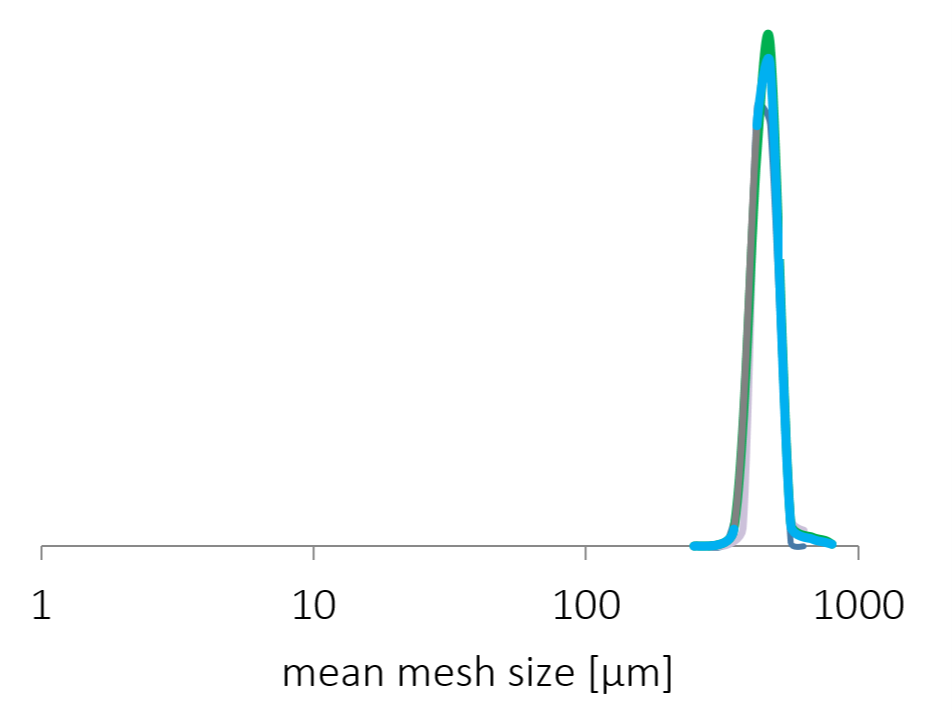
Size distribution of MCC pellets, type CELLETS® 350.
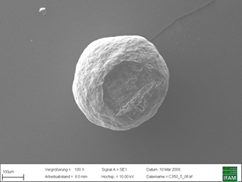
Electron microscopy image of a CELLETS® 350 starter beads. A high degree of sphericity and a smooth surface are advantages of these starter beads.

Finalized hydrocortisone pellets. Embedded pictures: electron microscopy image of a hydrocortisone pellet (top) and cross section (bottom).
After processing the hydrocortisone pellets are finalized and look – as the CELLETS® starter beads do – perfectly spherical with a smooth surface (Figure 5).
Summary
In this case study hydrocortisone by means of use in paediatric population for children suffering from all forms of adrenal insufficiency was investigated. Based on CELLETS® 350 starter beads, multi-layering hydrocortisone pellets were manufactured with five dosage strengths between 0,5 mg and 10 mg. Extremely bitter API was successfully taste masked and a fast dissolution profile was obtained. Clinical trial material was produced for Phase I study.
Acknowledgement
We acknowledge Fraunhofer IFAM (Dresden, Germany) and University of Basel (Basle, Switzerland) for providing the electron microscopic images.
Authors
Dr. Bastian Arlt
References
[2] Hydrocortisone, https://www.drugs.com/ (2016)

 ingredientpharm
ingredientpharm