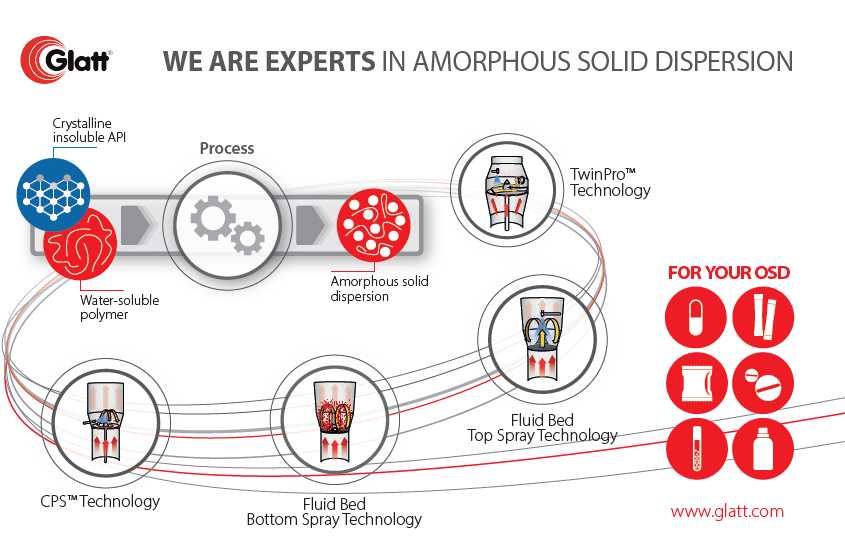
Introduction on amorphous solid dispersions
What is the benefit of multilayer amorphous solid dispersions? Recently, several studies had been performed on amorphous solid dispersions working spheres or starter beads. Starter beads, such as MCC (Microcrystalline Cellulose) spheres are employed due to their high friability and chemical inertness. Some studies are even working on solventless pelletization and amorphization using high shear granulator techniques [1].
Amorphization of poorly water-soluble drugs is a promising approach to improve the solubility and dissolution rate as amorphous solids lack a crystal lattice with long-range order [2]. Unfortunately, a high chemical potential compared to crystalline forms makes amorphous forms thermodynamically unstable. Thus, amorphous drugs exhibit low physical stability and finally lack of recrystallization [3,4]. In turn, surface crystallization is to be minimized.
Multilayer amorphous solid dispersions
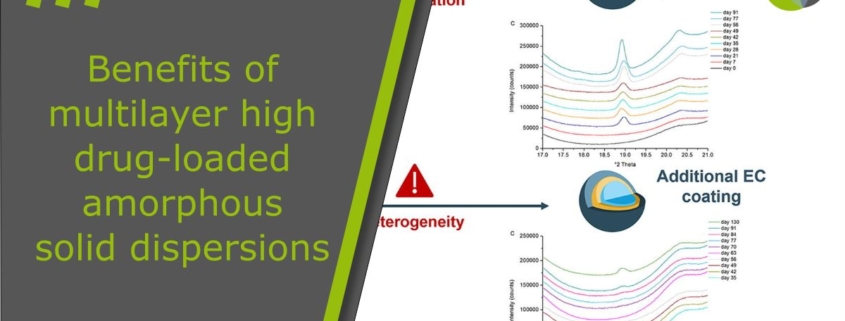
This is the key focus of a publication by Eline Boel and Guy Van den Mooter: They had been investigating a promising solution of multilayer high-drug load amorphous solid dispersions, as follows [5]:
Inhibiting surface crystallization is an interesting strategy to enhance the physical stability of amorphous solid dispersions (ASDs), still preserving high drug loads. The aim of this study was to investigate the potential surface crystallization inhibitory effect of an additional polymer coating onto ASDs, comprising high drug loads of a fast crystallizing drug, layered onto pellets. For this purpose, bilayer coated pellets were generated with fluid-bed coating, of which the first layer constitutes a solid dispersion of naproxen (NAP) in poly(vinylpyrrolidone-co-vinyl acetate) (PVP-VA) in a 40:60 or 35:65 (w/w) ratio, and ethyl cellulose (EC) composes the second layer. The physical stability of these double-layered pellets, in comparison to pellets with an ASD layer only, was assessed under accelerated conditions by monitoring with X-ray powder diffraction (XRPD) at regular time intervals. Bilayer coated pellets were however found to be physically less stable than pellets with an ASD layer only. Applying the supplementary EC coating layer induced crystallization and heterogeneity in the 40:60 and 35:65 (w/w) NAP-PVP-VA ASDs, respectively, attributed to the initial contact with the solvent. Caution is thus required when applying an additional coating layer on top of an ASD layer with fluid-bed coating, for instance for controlled release purposes, especially if the ASD consists of high loads of a fast crystallizing drug.
Read more on doi:10.1016/j.ijpharm.2022.122455.
How about following up studies on ASD formulation with starter beads? Simply, contact us für MCC spheres, such as CELLETS® 700 (700-1000 µm, US mesh 18/25).
Your technology and formulation partner for amorphous solid dispersions:
References
[1] K. Kondo, T. Rades, European Journal of Pharmaceutics and Biopharmaceutics 181 (2022) 183–194 doi:10.1016/j.ejpb.2022.11.011
[2] B.C. Hancock, M. Parks, Pharm. Res. 17 (2000) 397-404.
[3] L.I. Blaabjerg, E. Lindenberg, T. Rades, H. Grohganz, K. Lobmann, Int. J. Pharm. 521 (2017) 232-238.
[4] A. Singh, G. Van den Mooter, Adv. Drug Deliv. Rev. 100 (2016) 27-50.
[5] E. Boel, G. Van den Mooter, International Journal of Pharmaceutics (2022) 122455. doi:10.1016/j.ijpharm.2022.122455


 ingredient pharm
ingredient pharm  ingredientpharm
ingredientpharm 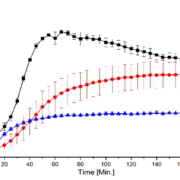

 ingredient pharm
ingredient pharm