The publication by G. Kumisbek, D. Vetchý, A. Kadyrbay describes gently the development and implementation of a new generic enteric form of CELLETS® 700 (MCC pellets, 700-1000 µm) loaded with omeprazole in capsules by Viva Pharm LLP, a pharmaceutical company based in Kazakhstan. The company’s solid-dosage-form production facility was utilized for this purpose. Detailed technological parameters for production were established and documented in production protocols for commercial batches. Stability testing confirmed the viability of the developed drug in two types of packaging. An analytical normative document containing comprehensive quality control test methods was created for both experimental purposes and the registration dossier.
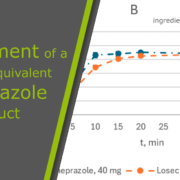
Equivalence to the innovative drug, Losec®, was demonstrated through dissolution profile testing and Bioequivalence Study (BES). The aim is to commercialize the new generic drug in the pharmaceutical market of Kazakhstan upon obtaining marketing authorization. This initiative aligns with Kazakhstan’s strategy to localize pharmaceutical production, mirroring similar practices in Europe, to ensure affordable and high-quality medicines for its population. The successful development and localization of this generic omeprazole formulation signify a step towards enhancing access to essential medications within Kazakhstan.
Abstract
Background and Objectives: The enteric form of omeprazole is one of the most commonly prescribed medications. Similarly to Europe, Kazakhstan relies on the localization of pharmaceutical drug production as one of its primary strategies to ensure that its population has access to affordable and good-quality medicines. This study comprehensively describes the technologically available development of bioequivalent delayed-release omeprazole. Materials and Methods: Various regimes and technological parameters were tested on laboratory- and production-scale equipment to establish a technical process where a functional and gastro-protective layer is essential. According to the ICH guidance on stability testing and Kazakhstan local rules, stability studies were conducted under conditions appropriate for climate zone II. The comparison of the rate and extent of absorption with subsequent assessment of the bioequivalence of the generic and reference drugs after a single dose of each drug at a dose of 40 mg was performed. Results: The quantitative and qualitative composition and technology of producing a new generic enteric form of omeprazole in capsules were developed and implemented at the manufacturing site of solid forms. Dissolution profiles in media with pH 1.2 and 6.8 were proven. During the accelerated six-month and long-term twelve-month studies, the developed formulation in both packaging materials at each control point passed the average weight and mass uniformity test, dissolution test, acid-resistance stage test, buffer stage test, impurity assay, and microbiological purity test and met all the specification criteria. A bioequivalence study in 24 healthy volunteers compared against the innovative drug showed the bioequivalency of the new generic system. The obtained values from the test and reference products were 1321 ± 249.0 ng/mL and 1274 ± 233 ng/mL for Cmax, 4521 ± 841 ng·h /mL and 4371 ± 695 ng·h /mL for AUC0-t, and 4636 ± 814 ng·h /mL and 4502 ± 640 ng·h /mL for AUC0-∞. Conclusions: Using affordable technologies, a bioequivalent generic delayed-release formulation of 20 and 40 mg omeprazole has been developed.
Materials
The following materials were used in the study: omeprazole (Hetero Labs, Hyderabad, India) Active Pharmaceutical Ingredient (API), lactose monohydrate Supertab 22AN (Glentnam Life Sciences Ltd., Corsham, UK), sodium lauryl sulfate (RNDr Kulich Pharma s.r.o., Hradec Králové, Czech Republic), disodium hydrogen phosphate dodecahydrate cryst. EMPROVE® (Merck KGaA, Darmstadt, Germany), hydroxypropylmethylcellulose (Tailopur 603), hydroxypropyl cellulose Klucel EF Pharm (Ashland Industries Europe GmbH, Schaffhausen, Switzerland), pellets from microcrystalline cellulose (MCC) Cellets®700 (International Process center GmbH & Co. KG, Dresden, Germany), polyethylene glycol 400 (Applichem GmbH, Darmstadt, Germany), methacrylic acid–ethyl acrylate copolymer Eudragit® L30-D55 (Evonic Nutrition and Care GmbH, Darmstadt, Germany), hard gelatin capsules size 0 (Capsugel, Bornem, Belgium), PlasAcryl® HTP20 (Emerson Resources Ink., Norristown, PA, USA), titanium dioxide (Venator Germany GmbH, Krefeld, Germany), talc (Imerys Talc Italy S.p.A, Porte, Italy). All ingredients were of pharmaceutical production grade as described in the European Pharmacopoeia, and are widely used in preparations for oral use at concentrations not exceeding the recommended limits. Losec®, enteric capsules, 20 mg, AstraZeneca AB, Sweden, was used as the reference drug.
Conclusion
The quantitative and qualitative composition and technology for producing a new generic enteric form of omeprazole in capsules were developed and implemented at the solid-dosage-form production facility of the company Viva Pharm LLP, located in Kazakhstan. The technological parameters of the production process were established and specified in production protocols for commercialized product batches. The developed drug in two types of packaging has passed the stability test. An analytical normative document consisting of full quality control test methods was created for the experimental part and the registration dossier. The equivalence of the generic formulation omeprazole Viva Pharm, enteric capsules, to the innovative drug Losec®, enteric capsules, was proved by the dissolution profile test and BES. The new generic drug is aimed to be commercialized in the pharmaceutical market of the Republic of Kazakhstan after obtaining marketing authorization. Similarly to Europe, Kazakhstan relies on the localization of pharmaceutical drug production as one of its primary strategies to ensure that its population has access to affordable and high-quality medicines.
Source: Medicina 2024, 60(3), 427

 ingredientpharm
ingredientpharm