Abstract
This case study is a short abstract of the publication by N. Scott et al. [1].
The dissolution kinetics of drugs in the environment of intestinal fluids is generally modelled by phosphate and physiological bicarbonate buffer solutions. Purged gases such as nitrogen (N2) and carbon dioxide (CO2) are used to stabilize the pH of bicarbonate buffers. As a matter of fact, disturbing hydrodynamics of gas purging limit this method.
This case study will show an attempt, how pH stabilization and minimized hydrodynamics can be realized simultaneously by suppling gases above the surface of the dissolution medium in the vessel. Drug release profiles of enteric coated microparticles, pellets (Cellets® 100 and Cellets® 1000, IPC Dresden, Germany) and tablets are investigated under novel conditions of gas supply in two different buffers.
Formulation of prednisolone-loaded microparticles and pellets
Prednisolone was layered onto microcrystalline cellulose cores using a fluid bed coater (Mini-Glatt, Glatt GmbH, Germany). The drug-loading suspension contained prednisolone, hypro-mellose, talc and deionized water (Table 1).
| Material | Composition |
| prednisolone | 9.79% w/w |
| hypromellose | 1.04% w/w |
| talc | 1.83% w/w |
| deionized water | 87.34% w/w |
Table 1: Formulation of prednisolone layered starter pellets of microcrystalline cellulose (Cellets®).
In order to reach a desired drug release profile, prednisolone layered Cellets® were coated with an Eudragit® (L30 D-55) dispersion. Cellets® 100 interacting as microparticles and Cellets® 1000 interacting as pellets showed a yield of 87 % and 100 %, respectively. The particle size of uncoated and polymer coated microparticles and pellets are given in Table 2.
| Material | D50 [µm] |
| Cellets 100® (microparticles) | |
| uncoated | 160.33 ± 2.09 |
| polymer coated | 216.94 ± 0.48 |
| Cellets 1000® (pellets) | |
| uncoated | 1140 ± 50 |
| polymer coated | 1199 ± 23 |
Table 2: Cellets® size of uncoated pellets and polymer-coated pellets with Eudragit® (L30 D-55).
A comparison of a coated pellet and a coated microparticle is shown in Figure 1. For further comparison of the dissolution dynamics, commercially available tablets (batch # PW242, Actavis Group PTC, UK) are taken. The composition of tablets is described in [1].
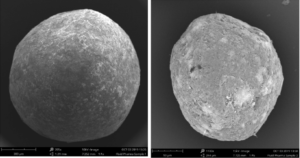
Figure 1: Electron microscopy images of a coated pellet (Cellets® 1000, left), and a coated microparticle (Cellets® 100, right).
Drug release profile
The drug release profile was measured for all three specimens – microparticles, pellets and tablets, as well. The starting stage is characterized by an acidic media (0.1 M HCl, pH < 1), while after two hours, the media was switched into buffer stages at pH 6.8 (phosphate buffer or bicarbonate buffer) by titration for another 2 hours. It is shown, that further N2/CO2 gas supply through a novel enclosure device is realized for the bicarbonate buffer with a precise pH-control with an accuracy better than 2 % and no disturbance to the hydrodynamics of the media.
Figure 2 and Figure 3 present the dissolution curve versus time from enteric coated prednisolone formulations. As expected, the dissolution in acidic media for microparticles and pellets is almost zero, while the drug release in the basic solution is tremendously higher and reaches a desired dissolution of 85 % after 5-20 minutes in a phosphate buffer (pH 6.8), and 15-65 minutes in a bicarbonate buffer (pH 6.8), respectively.
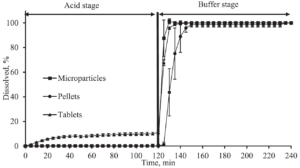
Figure 2: Dissolution versus time from enteric coated prednisolone formulations. The acidic stage is present by a 0.1 M HCl solution for 2 hours, subsequently is switched to a phosphate buffer at pH 6.8 with a duration of 2 more hours.
Formerly known obstacles with pH stabilities which are typical for bicarbonate buffers, was counteracted with controlled gas supply.
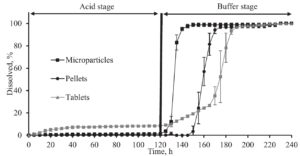
Figure 3: Dissolution versus time from enteric coated prednisolone formulations. The acidic stage (0.1 M HCl) is kept for 2 hours, subsequently is switched to a bicarbonate buffer at pH 6.8 with a duration of 2 hours.
Summary
This case study highlights the dissolution characteristics of prednisolone layered microcrystalline cellulose pellets. Phosphate and bicarbonate buffers have been used as in vitro tool for modelling the time dependent drug release. By using a novel gas purging technique onto the media surface, a better pH stability for the bicarbonate buffer was obtained and no foaming was observed. This innovative dissolution system is an important step targeting the replacement of phosphate buffers in dissolution models.
The new dissolution system enabled the comparison of enteric coated microparticles with pellets and tablets in bicarbonate buffers. The Cellets® 100 microparticles showed much faster drug release in the bicarbonate buffer than tablets and Cellets® 1000 pellets, indicating potential improvement in in vivo performance.
Acknowledgement
Dr. Fang Liu and her team are gratefully acknowledged for serving content and data for this note.
Information on Fluid Pharma:
Fluid Pharma Ltd
Contact: Dr. Fang LIU