Multiparticulate oral dosage form of tapentadol introduces a modern way to control drug release and improve pain management. The invention, described in patent US20250295596A1, replaces traditional monolithic extended-release tablets with numerous coated particles. This structure allows a smoother and more consistent release of tapentadol in the body. As a result, patients experience steadier pain relief, better compliance, and fewer side effects caused by fluctuating drug levels.
Key Findings of the Patent
The patent describes a system built from coated particles that contain tapentadol at the core. Each particle has a polymer and lubricant coating that controls how fast the drug is released. The combination of cellulose or acrylate polymers with magnesium stearate slows down the release effectively. In addition, the inventors found that high amounts of lubricant can support long-lasting release without affecting stability.
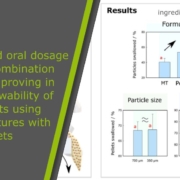
Unlike older tablet systems, this multiparticulate oral dosage form of tapentadol needs no extra subcoat between the drug and the coating layer. Therefore, manufacturing becomes easier and faster. Moreover, the system can include both immediate-release and extended-release particles. This design creates bimodal or multimodal kinetics, giving patients quick pain relief followed by prolonged action. The release rate can also be fine-tuned by adjusting coating thickness or lubricant particle size.
Importance for Human Health
This multiparticulate oral dosage form of tapentadol offers many advantages for patients. The small coated particles are easier to swallow than large tablets. Once in the body, they spread evenly through the digestive tract. This even distribution reduces irritation and ensures steady absorption. As a result, patients benefit from consistent pain control and fewer peaks or drops in drug concentration.
Furthermore, the formulation resists alcohol-induced dose dumping, which improves safety for opioid treatments. Because of its stability and flexibility, manufacturers can produce it reliably and at scale. This robust performance enhances both patient safety and production efficiency.
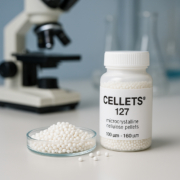
Role of CELLETS® 350 as Excipient
CELLETS® 350 serve as the excipient cores in this multiparticulate oral dosage form of tapentadol. These spherical microcrystalline cellulose pellets act as starter materials for layering the drug. They are uniform, strong, and chemically neutral. Thanks to their smooth surface and precise size, CELLETS® 350 allow a very even coating of tapentadol. This uniformity is crucial for predictable drug release. In addition, their good flow properties make manufacturing faster and more consistent. Therefore, Cellets 350 improve both the quality and efficiency of the formulation process.
Conclusion
The multiparticulate oral dosage form of tapentadol marks an important step forward in pain management. It combines precise control of drug release with easier swallowing and safer use. The use of CELLETS® 350 as excipient cores ensures reliable layering and coating, leading to consistent performance. Overall, this new dosage form provides a patient-friendly, safe, and scalable solution that improves therapeutic outcomes and production efficiency.
Patent Details
- Name or patent: Multiparticulate oral dosage form providing prolonged release of tapentadol
- Patent number: US20250295596A1
- Year of patent: 2025
- Patent holder names and affiliation: Marc Schiller, Ulrich Reinhold, Ulrike Bertram, Wolfgang Prange, Anika-Anina Philipp, Stefanie Straub, Annette Grave, Norbert Poellinger