Abstract
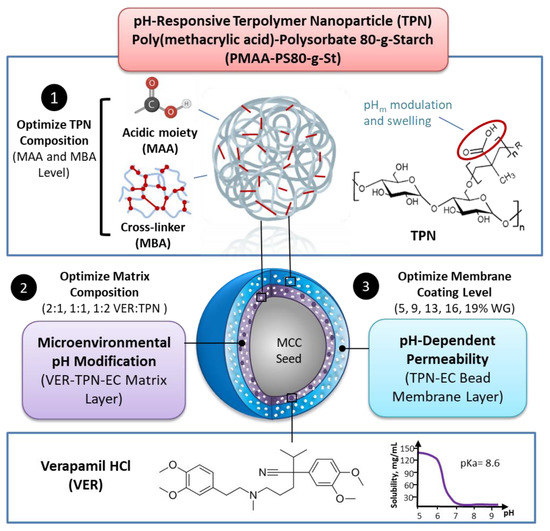
Bioavailability of weakly basic drugs may be disrupted by dramatic pH changes or unexpected pH alterations in the gastrointestinal tract. Conventional organic acids or enteric coating polymers cannot address this problem adequately because they leach out or dissolve prematurely, especially during controlled release applications. Thus, a non-leachable, multifunctional terpolymer nanoparticle (TPN) made of cross-linked poly(methacrylic acid) (PMAA)-polysorbate 80-grafted-starch (PMAA-PS 80-g-St) was proposed to provide pH transition-independent release of a weakly basic drug, verapamil HCl (VER), by a rationally designed bilayer-coated controlled release bead formulation. The pH-responsive PMAA and cross-linker content in the TPN was first optimized to achieve the largest possible increase in medium uptake alongside the smallest decrease in drug release rate at pH 6.8, relative to pH 1.2. Such TPNs maintained an acidic microenvironmental pH (pHm) when loaded in ethylcellulose (EC) films, as measured using pH-indicating dyes. Further studies of formulations revealed that with the 1:2 VER:TPN ratio and 19% coating weight gain, bilayer-coated beads maintained a constant release rate over the pH transition and exhibited extended release up to 18 h. These results demonstrated that the multifunctional TPN as a pHm modifier and pH-dependent pore former could overcome the severe pH-dependent solubility of weakly basic drugs.
Introduction
Materials and Methods
2.1. Materials
Conclusions
Disclaimer
References
- Dvořáčková, K.; Doležel, P.; Mašková, E.; Muselík, J.; Kejdušová, M.; Vetchý, D. The effect of acid pH modifiers on the release characteristics of weakly basic drug from hydrophlilic-lipophilic matrices. AAPS Pharmscitech 2013, 14, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Bassi, P.; Kaur, G. pH modulation: A mechanism to obtain pH-independent drug release. Expert Opin. Drug Deliv. 2010, 7, 845–857. [Google Scholar] [CrossRef]
- Sun, Y.; Koyama, Y.; Shimada, S. Measurement of intraluminal pH changes in the gastrointestinal tract of mice with gastrointestinal diseases. Biochem. Biophys. Res. Commun. 2022, 620, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Vinarov, Z.; Abdallah, M.; Agundez, J.A.G.; Allegaert, K.; Basit, A.W.; Braeckmans, M.; Ceulemans, J.; Corsetti, M.; Griffin, B.T.; Grimm, M.; et al. Impact of gastrointestinal tract variability on oral drug absorption and pharmacokinetics: An UNGAP review. Eur. J. Pharm. Sci. 2021, 162, 105812. [Google Scholar] [CrossRef] [PubMed]
- Thoma, K.; Ziegler, I. The pH-independent release of fenoldopam from pellets with insoluble film coats. Eur. J. Pharm. Biopharm. 1998, 46, 105–113. [Google Scholar] [CrossRef]
- Bolourchian, N.; Dadashzadeh, S. PH-independent release of propranolol hydrochloride from HPMC-based matrices using organic acids. Daru 2008, 16, 136–142. [Google Scholar]
- Nie, S.; Pan, W.; Li, X.; Wu, X. The effect of citric acid added to hydroxypropyl methylcellulose (HPMC) matrix tablets on the release profile of vinpocetine. Drug Dev. Ind. Pharm. 2004, 30, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, R.; Hong, E.; Villafuerte, L. Influence of admixed citric acid on the release profile of pelanserin hydrochloride from HPMC matrix tablets. Int. J. Pharm. 2000, 201, 165–173. [Google Scholar] [CrossRef]
- Streubel, A.; Siepmann, J.; Dashevsky, A.; Bodmeier, R. pH-independent release of a weakly basic drug from water-insoluble and -soluble matrix tablets. J. Control. Release 2000, 67, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Obaidat, A. Modulation of the micro-environmental pH and its influence on the gel layer behavior and release of theophylline from hydrophilic matrices. Int. J. Pharma. Bio Sci. 2013, 4, P794–P802. [Google Scholar]
- Siepe, S.; Lueckel, B.; Kramer, A.; Ries, A.; Gurny, R. Assessment of tailor-made HPMC-based matrix minitablets comprising a weakly basic drug compound. Drug Dev. Ind. Pharm. 2008, 34, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Siepe, S.; Lueckel, B.; Kramer, A.; Ries, A.; Gurny, R. Strategies for the design of hydrophilic matrix tablets with controlled microenvironmental pH. Int. J. Pharm. 2006, 316, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Yoshioka, M.; Horibe, H.; Hirai, S.; Kitamori, N.; Toguchi, H. pH-independent controlled-release microspheres using polyglycerol esters of fatty acids. J. Pharm. Sci. 1994, 83, 1600–1607. [Google Scholar] [CrossRef]
- Lecomte, F.; Siepmann, J.; Walther, M.; MacRae, R.J.; Bodmeier, R. Blends of enteric and GIT-insoluble polymers used for film coating: Physicochemical characterization and drug release patterns. J. Control. Release 2003, 89, 457–471. [Google Scholar] [CrossRef]
- Sakellariou, P.; Rowe, R.C.; White, E.F.T. Polymer/polymer interaction in blends of ethyl cellulose with both cellulose derivatives and polyethylene glycol 6000. Int. J. Pharm. 1986, 34, 93–103. [Google Scholar] [CrossRef]
- Sakellariou, P.; Rowe, R.C. Phase separation and morphology in ethylcellulose/cellulose acetate phthalate blends. J. Appl. Polym. Sci. 1991, 43, 845–855. [Google Scholar] [CrossRef]
- Lecomte, F.; Siepmann, J.; Walther, M.; MacRae, R.J.; Bodmeier, R. pH-Sensitive polymer blends used as coating materials to control drug release from spherical beads: Elucidation of the underlying mass transport mechanisms. Pharm. Res. 2005, 22, 1129–1141. [Google Scholar] [CrossRef]
- Khan, M.Z.; Prebeg, Z.; Kurjaković, N. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers. I. Manipulation Of drug release using Eudragit L100-55 and Eudragit S100 combinations. J. Control. Release 1999, 58, 215–222. [Google Scholar] [CrossRef]
- John, R.; Howard, P.T. Controlled Release Formulation. U.S. Patent US4792452A, 20 December 1988. [Google Scholar]
- Amighi, K.; Timmermans, J.; Puigdevall, J.; Baltes, E.; Moës, A.J. Peroral sustained-release film-coated pellets as a means to overcome physicochemical and biological drug-related problems. I. In vitro development and evaluation. Drug Dev. Ind. Pharm. 1998, 24, 509–515. [Google Scholar] [CrossRef]
- Gruber, P.; Brickl, R.; Bozler, G.; Stricker, H. Dipyricamole Sustained Release Forms Comprising Lacquer-Coated Particles and the Preparation Thereof. U.S. Patent US4367217A, 4 January 1983. [Google Scholar]
- Cole, E.T.; Scott, R.A.; Connor, A.L.; Wilding, I.R.; Petereit, H.U.; Schminke, C.; Beckert, T.; Cadé, D. Enteric coated HPMC capsules designed to achieve intestinal targeting. Int. J. Pharm. 2002, 231, 83–95. [Google Scholar] [CrossRef]
- Felton, L.A.; Porter, S.C. An update on pharmaceutical film coating for drug delivery. Expert Opin. Drug. Deliv. 2013, 10, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Frohoff-Hülsmann, M.A.; Lippold, B.C.; McGinity, J.W. Aqueous ethyl cellulose dispersion containing plasticizers of different water solubility and hydroxypropyl methyl-cellulose as coating material for diffusion pellets II: Properties of sprayed films. Eur. J. Pharm. Biopharm. 1999, 48, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Luliński, P. Molecularly imprinted polymers based drug delivery devices: A way to application in modern pharmacotherapy. A review. Mater. Sci. Eng. C 2017, 76, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, M.; Mohammadi, A.; Manshadi, M.K.D.; Sanati-Nezhad, A. Mathematical and computational modeling of nano-engineered drug delivery systems. J. Control. Release 2019, 307, 150–165. [Google Scholar] [CrossRef]
- Al Ragib, A.; Chakma, R.; Dewan, K.; Islam, T.; Kormoker, T.; Idris, A.M. Current advanced drug delivery systems: Challenges and potentialities. J. Drug Deliv. Sci. Technol. 2022, 76, 103727. [Google Scholar] [CrossRef]
- Davoodi, P.; Lee, L.Y.; Xu, Q.; Sunil, V.; Sun, Y.; Soh, S.; Wang, C.-H. Drug delivery systems for programmed and on-demand release. Adv. Drug Deliv. Rev. 2018, 132, 104–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cheung, R.; Wu, X.Y.; Ballinger, J.R.; Bendayan, R.; Rauth, A.M. A study of doxorubicin loading onto and release from sulfopropyl dextran ion-exchange microspheres. J. Control. Release 2001, 77, 213–224. [Google Scholar] [CrossRef]
- Wong, H.L.; Bendayan, R.; Rauth, A.M.; Wu, X.Y. Development of solid lipid nanoparticles containing ionically complexed chemotherapeutic drugs and chemosensitizers. J. Pharm. Sci. 2004, 93, 1993–2008. [Google Scholar] [CrossRef]
- Shalviri, A.; Raval, G.; Prasad, P.; Chan, C.; Liu, Q.; Heerklotz, H.; Rauth, A.M.; Wu, X.Y. pH-Dependent doxorubicin release from terpolymer of starch, polymethacrylic acid and polysorbate 80 nanoparticles for overcoming multi-drug resistance in human breast cancer cells. Eur. J. Pharm. Biopharm. 2012, 82, 587–597. [Google Scholar] [CrossRef]
- Shalviri, A.; Chan, H.K.; Raval, G.; Abdekhodaie, M.J.; Liu, Q.; Heerklotz, H.; Wu, X.Y. Design of pH-responsive nanoparticles of terpolymer of poly(methacrylic acid), polysorbate 80 and starch for delivery of doxorubicin. Colloids Surf. B Biointerfaces 2013, 101, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chang, H.H.R.; Shalviri, A.; Li, J.; Lugtu-Pe, J.A.; Kane, A.; Wu, X.Y. Investigation of a new pH-responsive nanoparticulate pore former for controlled release enteric coating with improved processability and stability. Eur. J. Pharm. Biopharm. 2017, 120, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.I.; Gomes, E.C.L.; Soares, C.D.V.; Cunha, A.F.; Oliveira, M.A. Thermal Analysis Applied to Verapamil Hydrochloride Characterization in Pharmaceutical Formulations. Molecules 2010, 15, 2439–2452. [Google Scholar] [CrossRef] [PubMed]

 ingredientpharm
ingredientpharm 
 ingredientpharm
ingredientpharm