This article “Influence of Polymer Film Thickness on Drug Release from Fluidized Bed Coated Pellets and Intended Process and Product Control” was published on Pharmaceutics 2024, 16(10), 1307; https://doi.org/10.3390/pharmaceutics16101307, under free licence on October 08, 2024 by Marcel Langner, Florian Priese, and Bertram Wolf. We performed modifications of the text for better readability.
Abstract
Background/Objectives: Coated drug pellets are widely used in hard gelatine capsules. In heterogeneous pellets, the drug is layered onto core pellets. Coatings often provide retarded release or enteric protection.
Methods: In this study, we correlated polymer coating thickness on drug pellets with drug release kinetics.
Results: We investigated whether the coating process can be stopped once the desired layer thickness is achieved. First, inert pellets were coated with sodium benzoate. Then, they received different amounts of water-insoluble polyacrylate in a fluidized bed apparatus with a Wurster inlet. We controlled the entire process in-line and at-line using process analytical technology. This involved measuring particle size and layer thickness. Next, we examined in-vitro sodium benzoate release. We linearized the data with various standard models and compared it with polyacrylate layer thickness. As polyacrylate layer thickness increased, the release rate decreased. Several factors influenced release simultaneously, resulting in profiles that approximated first-order kinetics. Thus, the coating thickness corresponded to a specific drug release profile.
Conclusions: Manufacturing coated drug pellets with a targeted release is achievable through process control and layer thickness measurement. However, preliminary investigations are needed for each formulation.
1. Introduction
The dissolution of solid drug formulations depends on the solubility and dissolution rate of the drug substances. In addition, several factors influence release kinetics. For example, drug interaction with excipients, compression force and hardness in tablets, and the type of binder in granulates, pellets, or polymer coatings all play a role.
Understanding the dissolution rate and release kinetics is essential for optimal pharmacotherapy. Dissolution of solid substances typically follows first-order kinetics due to diffusion processes. However, some formulations show zero-order release, where equal amounts are released in equal time intervals.
Multiple processes often occur simultaneously. These include wetting of the dosage form, drug dissolution, diffusion of drug molecules out of the dosage form, swelling of matrix formulations, and water uptake by insoluble films. As a result, the kinetics may not fit simple zero-, first-, square-, or cubic-root order equations.
To evaluate the best approach, release data are linearized using various models. The coefficient of determination (CoD) of the linearized curve indicates the best fit and suggests which process likely dominates [1,2,3,4,5,6].
The model-independent parameters, difference factor f1 and similarity factor f2, are used to compare release profiles. f1 describes the relative error between two release profiles. It is calculated from the cumulative amounts released at a specific time T for a test and a reference formulation, or more generally, between any two formulations—for example, during drug development. On the other hand, f2 is based on the sum of squared deviations of the released drug amounts from the two profiles [4,5,7,8,9].
Focuses on drug-loaded pellets and their controlled release
Increasing attention has therefore focused on drug-loaded pellets and their controlled release. Specifically, this control is achieved by slowly swelling matrix systems or, alternatively, a final functional coating. Consequently, researchers have investigated the release of drugs from matrix pellets prepared by extrusion/spheronization and, moreover, coated with different amounts and types of insoluble ethylcellulose [10,11,12].
In addition, other studies report additional factors influencing drug release. For example, these include the filler type [13], the pH of the release fluid [4], storage conditions of the drug and methylcellulose matrix pellets [14], the amount of enteric polymer coating [15], and, finally, the salt concentration in the release fluid [16].
Further investigations examine the effects of talc and hydrogenated castor oil on the dissolution of metformin-loaded matrix pellets with an acrylic-based sustained-release coating [17]. Researchers also studied the sustained release of Lisinopril from mucoadhesive matrix pellets [18] and sinomenine hydrochloride from pellets produced using a novel whirlwind fluidized bed process [19].
Drug-layered inert pellets coated with polymer (heterogeneous pellets) were studied in order to assess the influence of release kinetics. Specifically, researchers investigated modifications of ethylcellulose coatings [20]; furthermore, they studied ethylcellulose mixed with polyvinylpyrrolidone (PVP) as a pore former [21], alternating layers of ethylcellulose and polyvinylacetate [22], various coating levels with final curing [23], and additionally, acetaminophen-layered sugar pellets coated with ethylcellulose [24]. Moreover, with polyacrylate coatings, drug release from layered pellets was delayed [7,25]. Therefore, changing the polymer type and layer thickness allowed control of the release rate over a wide range [8].
heterogeneous pellets coated with sodium benzoate
In our previous studies, heterogeneous pellets were manufactured using fluidized bed technology with a Wurster inlet. Initially, inert microcrystalline cellulose pellets (Cellets®175, median 170 µm), which offer a large specific surface area, were first coated with excipients as well as the water-soluble model drug sodium benzoate [26,27,28]. Consequently, these sodium benzoate (SB) pellets showed narrow particle size distribution, high sphericity, homogeneous layers, and additionally, rapid drug release. Subsequently, to achieve retarded release, the SB pellets were coated a second time with different amounts of ethylcellulose using the same fluidized bed technique [29]. As expected, the release rate decreased with increasing coating thickness. Moreover, the process was monitored in-line using spatial filter velocimetry (SFV) probes [27,28] to ensure control over particle size, distribution, and ethylcellulose layer thickness.
The present project aimed to produce heterogeneous pellets in a fluidized bed with a Wurster inlet while controlling the process using in-line particle size and coating thickness measurements. We studied sodium benzoate release kinetics, interpreted the partial processes affecting release, correlated release rate with polymer thickness, and determined the coating process endpoint to improve pellet quality.
PVP binder to improve layer stability
For pellet manufacturing, we followed a similar experimental approach to [26,27,28]. Small initial inert pellets (Cellets®175, median 170 µm) with large specific surface areas were coated with a sodium benzoate solution containing a small amount of PVP binder to improve layer stability. In the second step [29], SB pellets received varying amounts of insoluble but slowly swelling polyacrylate for retarded release. Agglomeration risk during coating was minimized by adjusting process parameters and adding talcum as an anti-stick agent. The SFV probe monitored particle size and detected agglomerates in real time.
Drug release was analyzed using zero-order, first-order, square-root, and cubic-root kinetic models. We identified the most likely release model by calculating area under the curve (AUC), dissolution efficiency (DE), and mean dissolution time (MDT), and by comparing the CoD of different models. We also calculated the difference factor f1 and similarity factor f2 to compare release profiles of different polyacrylate-layered pellet batches. Linearization works well for first-order kinetics. For other release profiles, nonlinear methods describe dissolution curves more accurately and reduce standard deviation in fitting parameters [30].
2. Materials and Methods
2.1. Materials
2.2. Formulation of Sodium Benzoate-Coated Pellets
| Content (%) | |
|---|---|
| Sodium benzoate | 32.6 |
| Microcrystalline cellulose | 65.3 |
| PVP | 1.6 |
| Talcum | 0.5 |
| 100.0 |
2.3. Formulation of polyacrylate-coated sodium benzoate pellets
In the second coating step, SB pellets were layered with polyacrylate in three concentration lots: P1 (11.1% w/w PVP), P2 (14.3% w/w), and P3 (17.6% w/w). Magnesium stearate and talcum were added to the coating fluid. They acted as a plasticizer and an anti-stick agent, respectively (Table 2).
The polyacrylate coating fluid contained 13.3% (w/w) polyacrylate copolymer, 1.3% (w/w) magnesium stearate, and 5.3% (w/w) talcum. To prepare the mixture, we added a Eudragit®NE 30D dispersion to a beaker. Next, magnesium stearate and talcum were added one after another. The dispersion was homogenized under strong agitation with a disperser (Ultra Turrax T50 standard, Janke & Kunkel, IKA Labortechnik, Staufen, Germany; length 225 mm, diameter 18 mm, rotation 5000 rpm).
| Lot | P1 | P2 | P3 |
|---|---|---|---|
| Content (%) | |||
| Sodium benzoate | 25.9 | 24.4 | 22.9 |
| Microcrystalline cellulose | 55.7 | 52.6 | 49.3 |
| PVP | 1.3 | 1.2 | 1.1 |
| Polyacrylate | 11.1 | 14.3 | 17.6 |
| Talcum | 4.9 | 6.1 | 7.4 |
| Magnesium stearate | 1.1 | 1.4 | 1.7 |
| 100.0 | 100.0 | 100.0 | |
2.4. Fluidized bed pellet coating
The coating process took place in a batch laboratory fluidized bed apparatus (GPCG 1.1, Glatt, Binzen, Germany). The system included a Wurster inlet and an SFV probe in the process chamber [27]. A spray nozzle of 1.0 mm diameter was used, with the nozzle cap set at 2.5 scales. The distance between the lower cylinder end and the perforated bottom plate B was fixed at 20 mm. The process air volume rate varied between 40 and 60 m³/h and was adapted to the increasing pellet weight during coating.
In the first step, Cellets®175 were coated with a sodium benzoate/PVP/talcum aqueous fluid (Table 3). In the second step, pellets received a polyacrylate dispersion under mild conditions. A lower spray rate and reduced process air temperature prevented pellet adhesion and sticking. Afterward, the polyacrylate-coated pellets were tempered for one hour at 30 °C. They were spread as a thin layer on a steel dish to allow coalescence and film formation.
| Parameter | First Step | Second Step |
|---|---|---|
| sodium benzoate | polyacrylate | |
| pellet batch (g) | 300 | |
| process air temperature (°C) | 80 | 40 |
| product temperature (°C) | 40 | 25 |
| process air volume rate (m3/h) | 40–60 | |
| spray rate (g/min) | 20 | 6 |
| spray pressure (bar) | 3 | |
2.5. Particle Size Coating Layer Thickness Measurement with SFV Probe
2.6. Sodium Benzoate Release and Content Investigation
The release was tested using a dissolution tester (PTW 2, Pharmatest, Hainburg). Specifically, the setup included six vessels containing 1.0 L purified water maintained at 37 °C, with a blade rotation of 75 rpm. Then, sampling took place at 10, 20, 30, 45, 60, 120, and 180 minutes. After each withdrawal, the volume was refilled with fresh purified water. Subsequently, sodium benzoate was analyzed using a UV–Vis spectrophotometer (Spekol 1300, Analytik Jena, Germany) with a 1 cm quartz cuvette at 220 nm.
For sodium benzoate content analysis, 50 mg of pellets were dispersed in 1.0 L purified water. These pellets contained 13.5 mg sodium benzoate. Dissolution and release were complete after 4 hours. The content was then analyzed as described above.
2.7. Linearization of Release Curves
The evaluation of the release curves was performed according to the different models of release kinetics also used by a number of authors [1,3,5,6,9,14]. In the first step of the release evaluation, the amount of cumulative released substance is plotted versus time. Linear curves arise in the case of zero order kinetics, i.e., equal amounts of the drug are released in equal time intervals (Equation (1)).
First-order release kinetics are typical for slightly soluble drugs in solid preparations such as tablets, pellets, and granules. These systems are dominated by slow dissolution and diffusion control. At the beginning of the process, the release rate is highest. This occurs because the large concentration gradient drives diffusion, as described in Fick’s first law (Equation 2). However, the release rate gradually decreases as the concentration gradient diminishes during the process.
The released amount Mt at the moment t refers to (Equation (3)), and linearization results in the Sigma minus function (Equation (4)).
The Weibull function (Equation (5)) and its linearized form (Equation (6)) presuppose first order kinetics.
Square root kinetics occurs at non-disintegrating solid matrix formulations (Equation (7)).
Cubic root kinetics are observed in the case of spherical multiparticulate formulations (linearized form, Equation (8)).
2.8. Model Independent Parameters: Difference Factor f1 and Similarity Factor f2
The difference factor f1 describes the relative error between two release profiles. It is calculated from the cumulative released amounts Ri and Ti at distinct time points for reference and test formulations (Equation 9). In contrast, the similarity factor f2 is based on the sum of squared deviations of released drug amounts (Equation 10). It expresses the statistical similarity between two profiles.
The f2 value equals 100 for identical profiles and ranges between 50 and 100 for similar ones. Together, both factors serve to compare the release profiles of generic and standard drug products. This comparison helps determine whether the generic profile matches or surpasses the standard.
In this study, we applied both factors to evaluate differences and similarities in sodium benzoate release profiles with various polymer coatings.
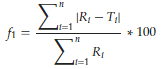
(9)
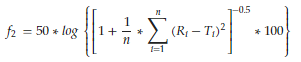
(10)
2.9. Microscopically Investigation
2.10. Sphericity
2.11. SFV Measurement
3. Results and Discussion
3.1. Properties of Sodium Benzoate and Polyacrylate Coated Pellets
SB pellets are received as a free-flowing material. The coating process proceeded smoothly, and the Wurster inlet created a homogeneous fluidized bed pattern. As a result, the product shows a narrow particle size distribution [27]. The median x50.3 increased from 170 µm (uncoated Cellets® 175) to 200 µm. The sphericity of both the initial Cellets®175 and SB pellets remained above 0.9.
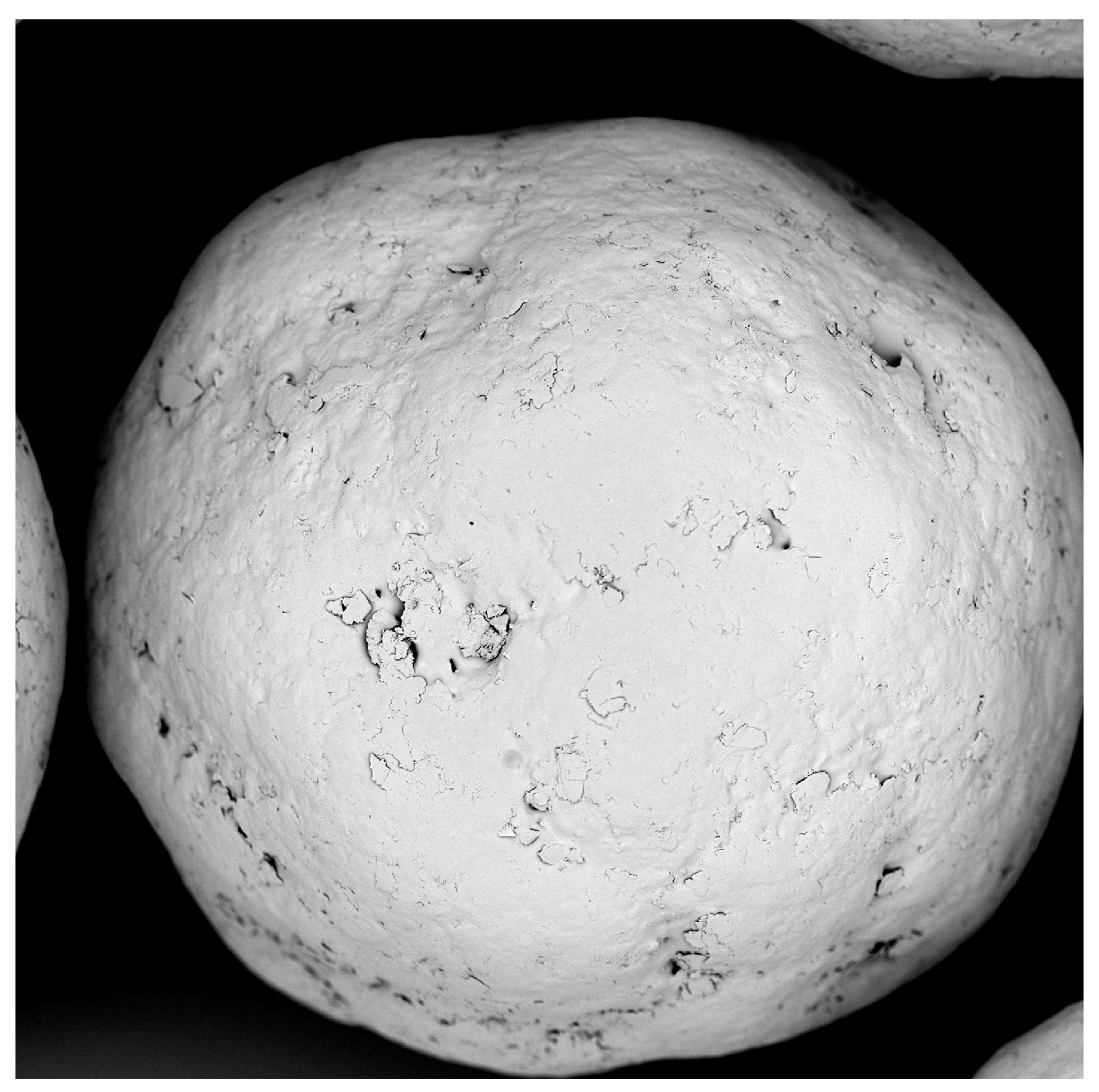
The polyacrylate coating of SB pellets caused no significant agglomeration. Only a few twins and triplets appeared under microscopic observation (Figure 1). The median size of polyacrylate-coated pellets grew to 232.2 µm, with a layer thickness of 16.1 µm (Table 4, P3, 17.6% polyacrylate content). Yield losses and incomplete sodium benzoate recovery resulted from material precipitation at the textile filter and inner chamber wall. Nevertheless, a sphericity above 0.9 confirms the formation of spherical products and indicates homogeneous processing.
| X50.3 (µm) | Polyacrylate Layer Thickness (µm) |
Yield (%) | Sodium Benzoate Content (%) |
Sphericity (-) |
|
|---|---|---|---|---|---|
| P1 | 213.0 | 6.5 | 84 | 92 | 0.91 |
| P2 | 221.0 | 10.5 | |||
| P3 | 232.2 | 16.1 |
3.2. Sodium Benzoate Release Kinetics
3.2.1. Double Linear Diagram (Zero Order Release Kinetics)
After five minutes, more than 90% of sodium benzoate dissolves from SB pellets without a polymer layer. This is due to its high solubility and rapid dissolution rate. Sodium benzoate behaves as a strong electrolyte (sodium salt of benzoic acid, pKa 4.19 [31]), so it dissociates considerably into sodium cations and benzoate anions.
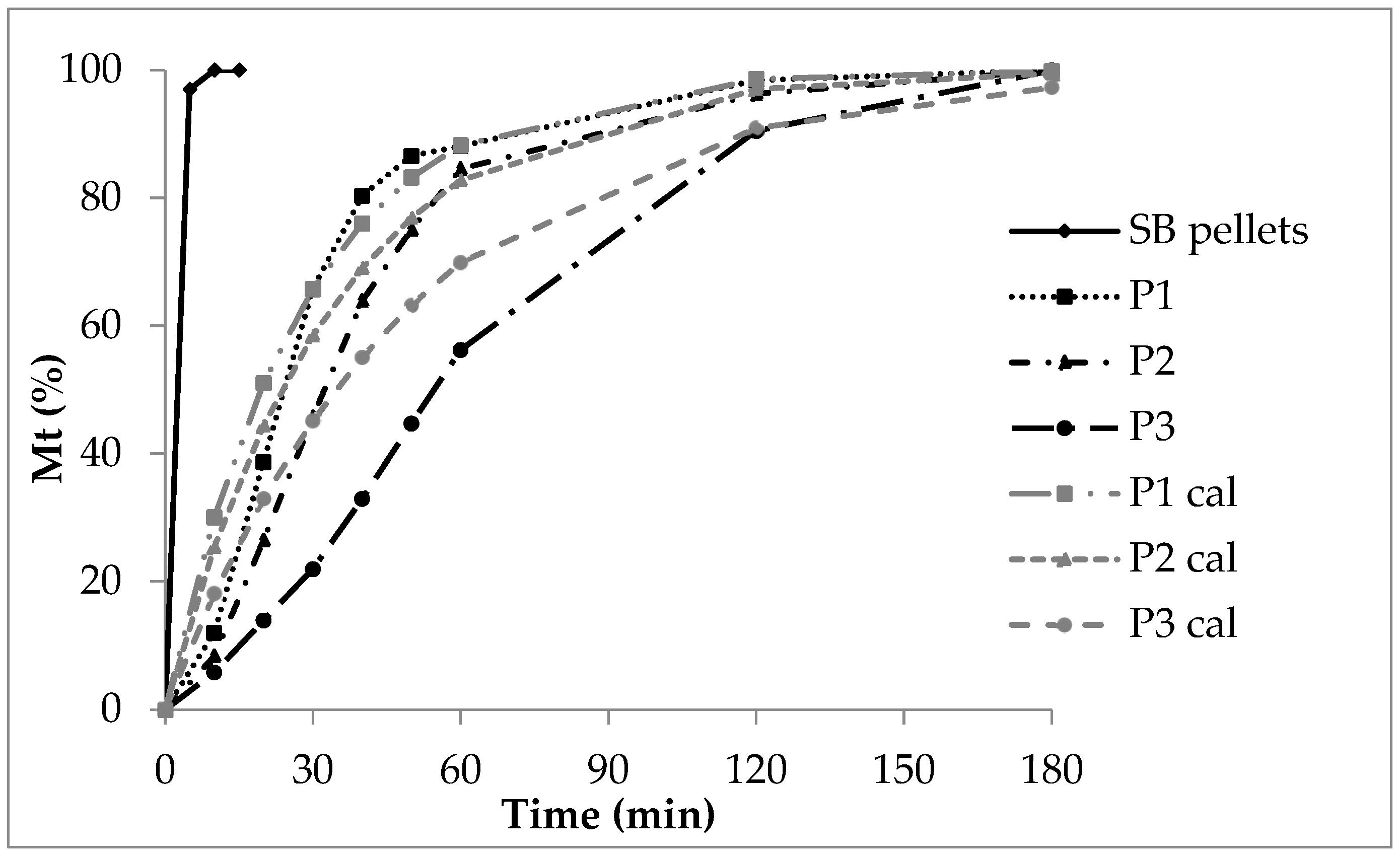
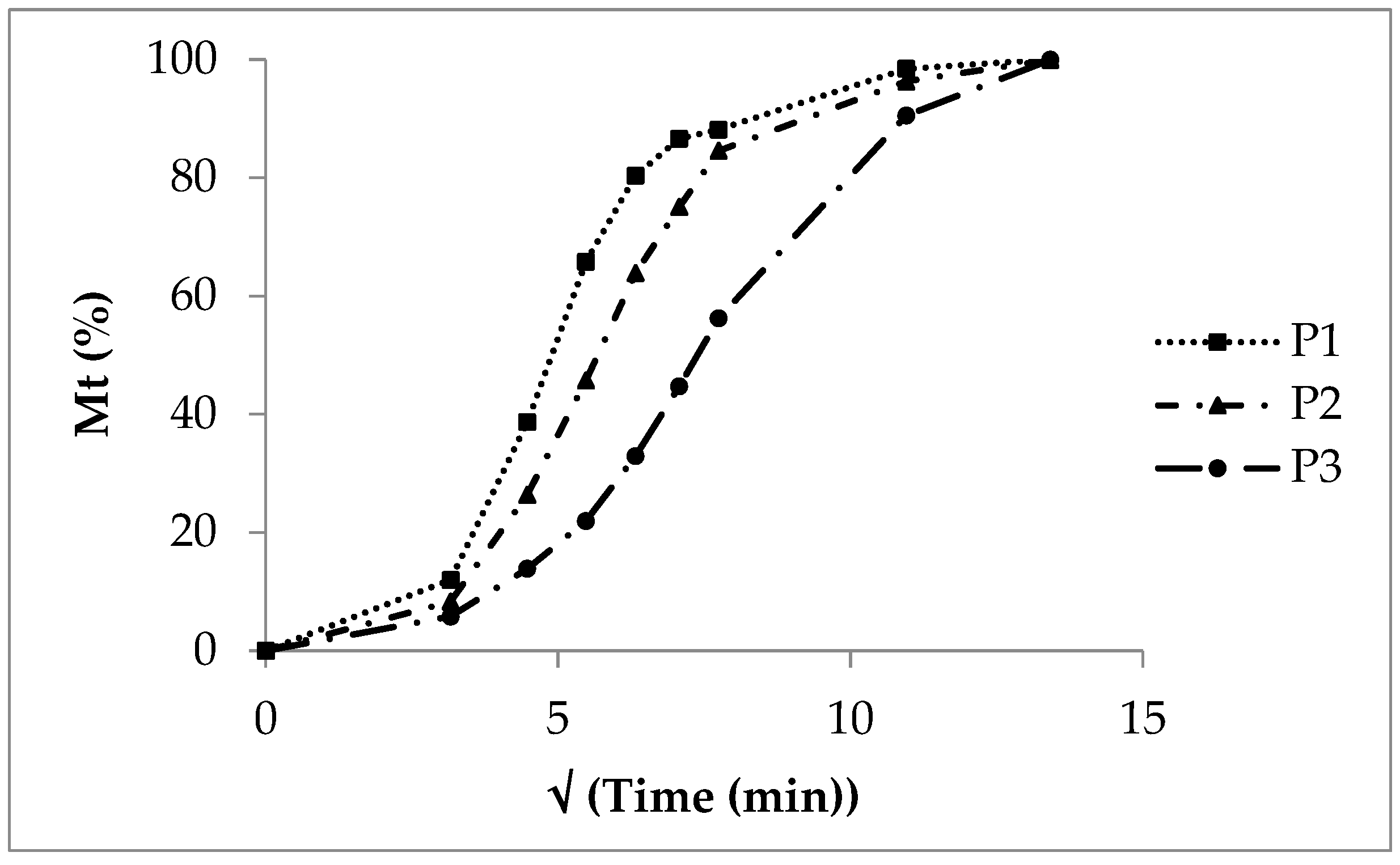
In contrast, the release from polyacrylate-coated pellets follows exponential curves (Figure 2). Generally, the release rate decreases as the polyacrylate layer thickens. The insoluble polyacrylate acts as a barrier. After ten minutes, 30% of sodium benzoate is released from low coating (P1), 20% from medium coating (P2), and 8% from high coating (P3).
diffusion of sodium benzoate molecules and ions
The dissolution rate of sodium benzoate alone cannot explain the release. Instead, diffusion of sodium benzoate molecules and ions through the polymer layer controls the rate. Initially, a high concentration gradient drives rapid release. Later, the release rate slows as the concentration gradient decreases.
For low coating (P1), the CoD of zero-order kinetics is 0.57 (Table 5), indicating zero-order release is unlikely. First-order diffusion seems to control the process. With increasing polyacrylate thickness, zero-order CoD rises (P2: 0.70, P3: 0.93). This reflects additional effects, such as polymer swelling and prolonged diffusion distance. Consequently, the release rate decreases as polyacrylate content rises, which is evident in decreasing AUC and DE, and increasing MDT (Table 6).
| CoD (R2) | |||
|---|---|---|---|
| Model | P1 | P2 | P3 |
| Zero order | 0.57 | 0.70 | 0.93 |
| First order Sigma minus | 0.98 | 0.98 | 0.95 |
| First order Weibull | 0.87 | 0.99 | 0.99 |
| Square root | 0.81 | 0.88 | 0.94 |
| Cubic root | 0.68 | 0.80 | 0.98 |
| AUC (%∗min) | DE (-) | MDT (min) | |
|---|---|---|---|
| P1 | 14,820 | 0.82 | 32 |
| P2 | 13,927 | 0.77 | 41 |
| P3 | 11,587 | 0.64 | 63 |
indicating equivalence between P1 and P2
| Parameter | Evaluation | P1/P2 | P1/P3 | P2/P3 |
|---|---|---|---|---|
| Difference factor f1 | “equivalent” 0–15 |
12 | 24 | 25 |
| Similarity factor f2 | “similar” 50–100 |
74 | 63 | 67 |
similarity factor f2 decreases with the increasing layer thickness
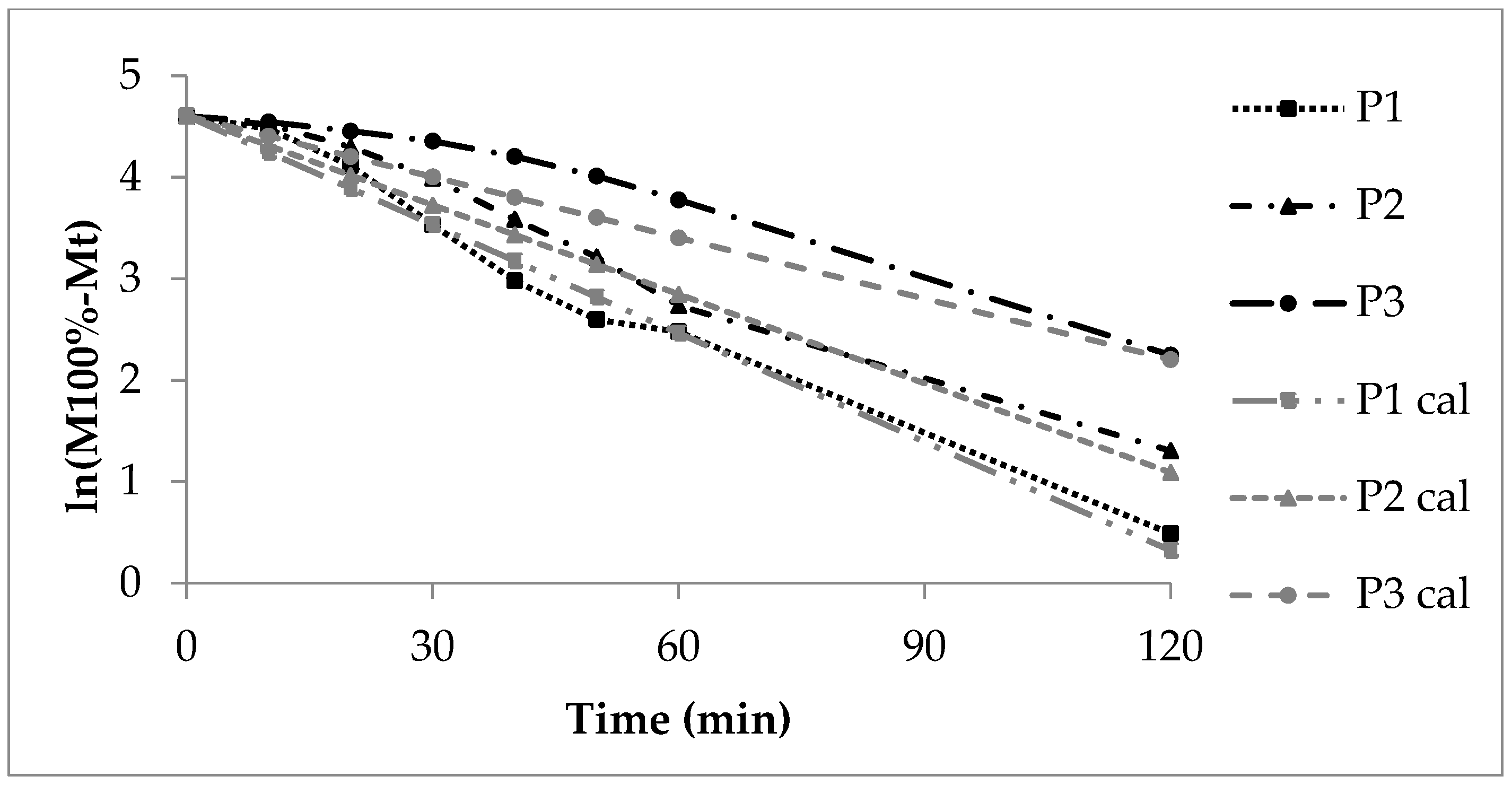
The calculated double linear curves (grey) roughly matched the experimental curves (black, Figure 2). However, the deviation was greatest for P3, which had the thickest polyacrylate layer. This was caused by several coinciding processes: slow film wetting and swelling, delayed water uptake, and limited diffusion through the polyacrylate film to the sodium benzoate layer. Next came sodium benzoate dissolution and its diffusion through the swollen polymer into the release fluid.
The high coating thickness played a critical role. It created a long diffusion path and continuously altered the sodium benzoate concentration gradient within the polyacrylate layer. Consequently, these factors strongly influenced the overall release rate.
3.2.2. First Order Kinetics, Sigma Minus Function
| k1 (1/min) Sigma Minus | b (-) Weibull |
1/a (-) Weibull |
t63.2% (min) Weibull |
|
|---|---|---|---|---|
| P1 | 0.036 | 1.08 | 0.25 | 30 |
| P2 | 0.030 | 1.58 | 0.17 | 40 |
| P3 | 0.020 | 1.36 | 0.17 | 70 |
3.2.3. First Order Kinetics, Weibull Function
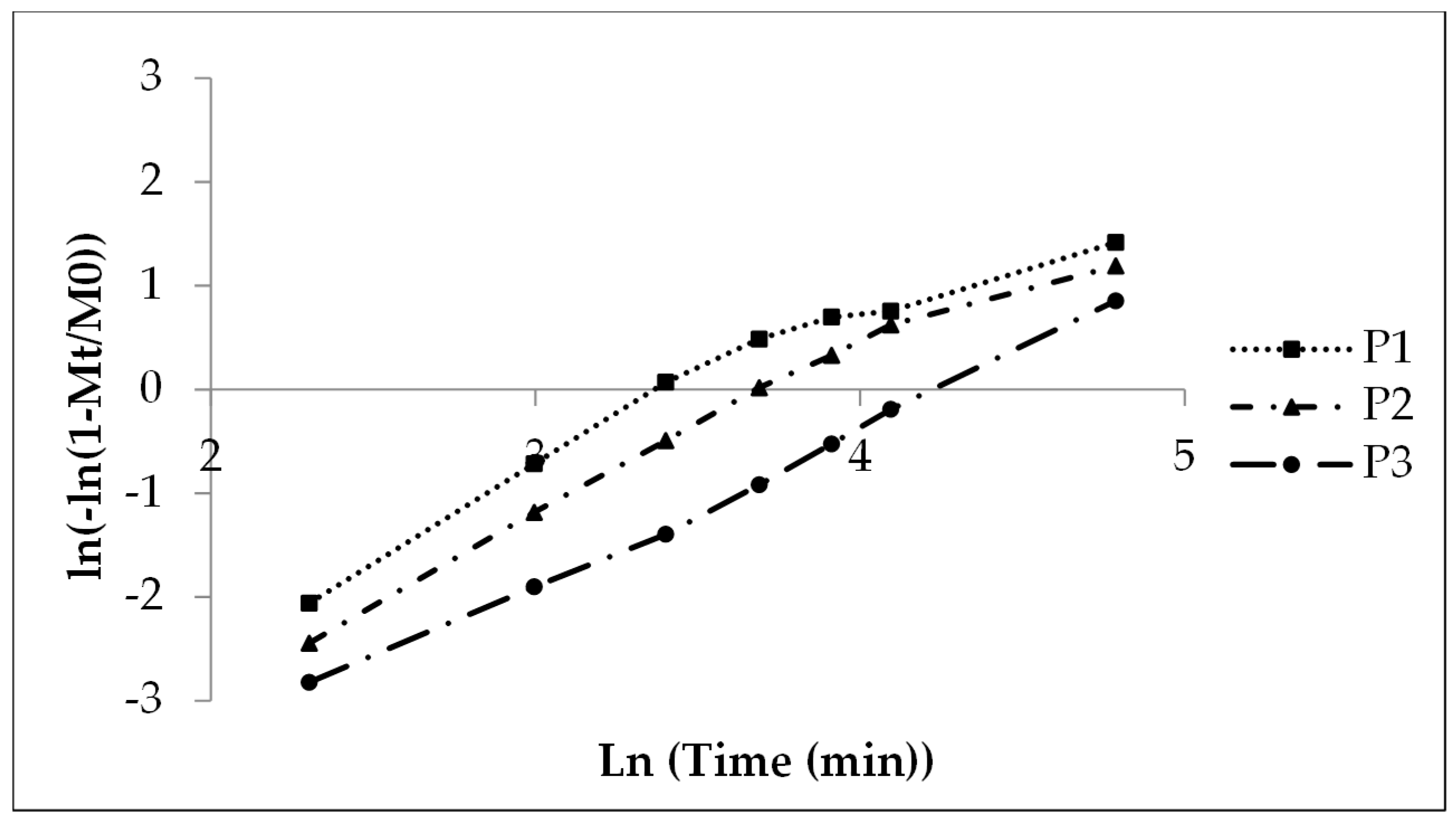
A shape parameter of 1 indicates monophasic release. In contrast, values above 1 suggest multiphasic release. In the present case, multiphasic release included an initial lag phase caused by wetting and swelling of the polyacrylate film. This was followed by an accelerated release rate up to the inflection point due to the high concentration gradient. Afterward, the rate slowed as the gradient decreased until drug dissolution and release were complete.
monophasic and multiphasic kinetics
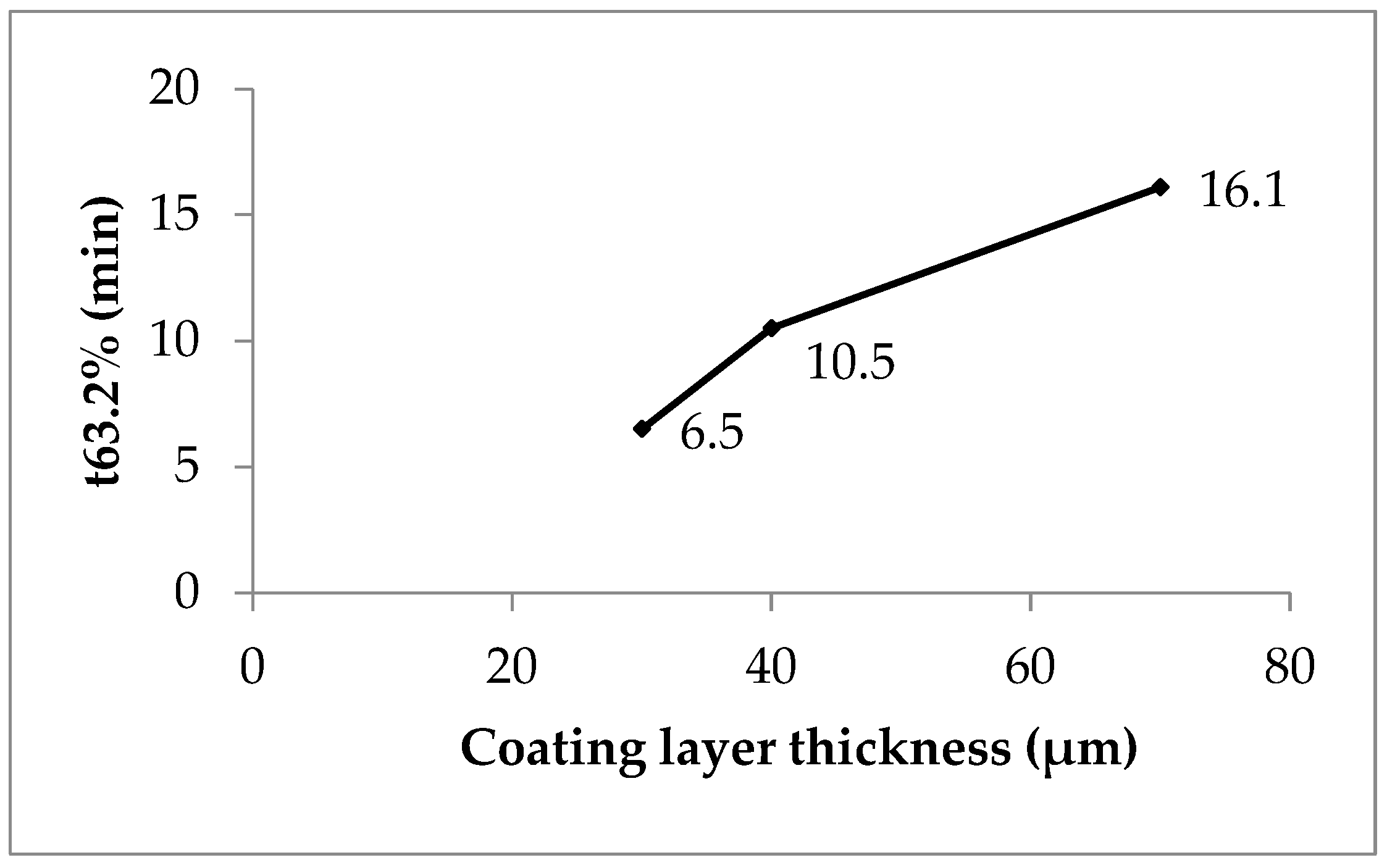
P1, with low coating, showed nearly monophasic kinetics (shape parameter 1.08, Table 8). However, P2 (1.58) and P3 (1.36) indicated a more pronounced multiphasic release. The scale parameter (1/a) refers to the rate constant. It decreased with increasing coating thickness (Table 8). The time parameter t63.2% marks the moment when 63.2% of sodium benzoate is released. This value increased with higher polyacrylate thickness, ranging from 30 to 70 minutes (Table 8, Figure 5; see also Figure 2).
Clearly, polyacrylate coating thickness strongly influenced release kinetics (Table 4). A practical strategy for manufacturing coated pellets with controlled release is as follows. First, prepare laboratory-scale lots with increasing coating thickness. Then, measure thickness with the SFV probe. Next, investigate in vitro release and correlate it with polymer thickness. Finally, in production scale, stop the coating process once the desired thickness is detected.
3.2.4. Square Root Function
A cumulative release plot versus the square root of time yields straight lines for diffusion-controlled release. This is typical for non-disintegrating matrices such as tablets and semisolid systems (ointments, creams). Lots P1 and P2 showed nearly straight lines between 10 and 60 minutes (Figure 6). However, the initial phase (up to 10 minutes) and the terminal phase (after 60 minutes) did not fit the square root model. The CoD values ranged from 0.81 for P1 to 0.94 for P3 (Table 5). Therefore, the square root model was not suitable to describe the release from pellets with an insoluble but swellable polymer coating.
3.2.5. Cubic Root Function
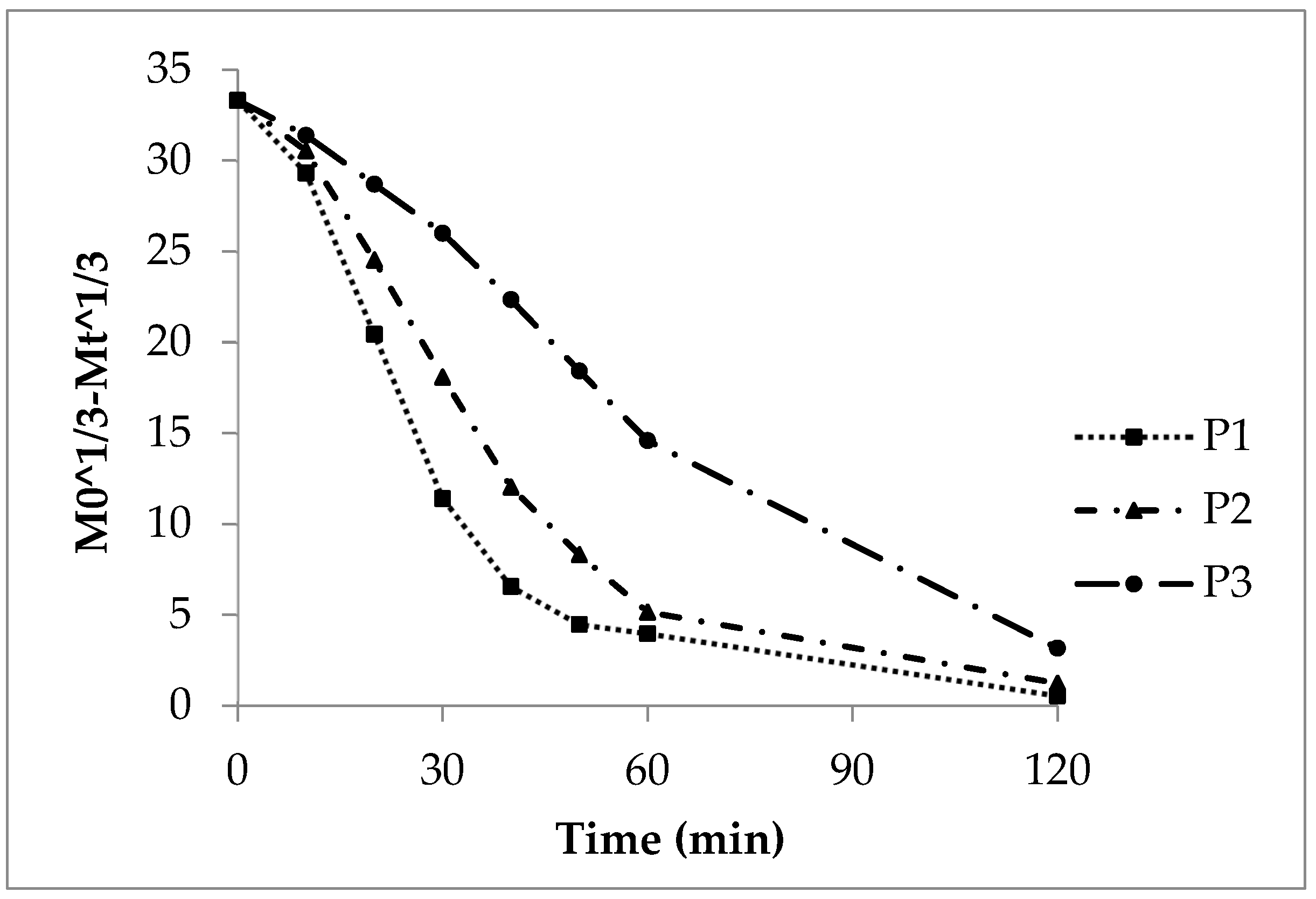
The cubic root function applies to the dissolution of spherical particles. This is because both weight and surface area decrease during dissolution. When the cubic roots of the dose and cumulative released substance are plotted against time, straight lines should appear.
However, this was not observed for sodium benzoate release from polyacrylate-coated pellets (Figure 7). The deviation from linearity was clear in lots P1 and P2, especially in the terminal release phase after 45 minutes. Their CoD values were 0.68 and 0.80, respectively (Table 5). In contrast, the slower-releasing P3 showed a nearly straight curve with a CoD of 0.98.
Thus, the cubic root model seems suitable only for pellets with thick polyacrylate coatings. Lots P1 and P2, with thinner coatings, did not follow cubic root kinetics or typical sphere dissolution.
Figure 7. Cubic root function of the experimental sodium benzoate release, lots P1, P2, and P3.
4. Conclusions
Inert Cellets® 175 were coated in two steps. First, they received the model drug sodium benzoate. Second, they were coated with a water-insoluble polyacrylate dispersion in a fluidized bed with a Wurster inlet. Particle size increase and coating thickness were measured in-line during the entire process using the SFV probe. Sodium benzoate release was then tested in vitro. The release profiles were linearized and evaluated with different kinetic models.
As the polyacrylate coating layer thickened, the sodium benzoate release rate decreased. This trend was confirmed by release parameters, rate constants, AUC, MDT, and DE. A difference factor f1 above 15 indicated dissimilar profiles between low-coated (P1, P2) and high-coated (P3) pellets. Thus, coating thickness significantly influenced sodium benzoate release. The similarity factor f2 ranged from 67 to 74, confirming comparable release profiles across lots P1, P2, and P3.
high CoD values
The high CoD values of linearized release profiles suggested first-order kinetics as the most suitable model. This outcome can be explained by the strong effect of sodium benzoate diffusion through the swollen polyacrylate film. With thicker coatings, polymer swelling increased. Consequently, diffusion distances for water and sodium benzoate grew longer, while concentration gradients exerted stronger control over release.
Overall, the detailed study of release profiles in relation to coating thickness allows accurate detection of the coating endpoint. Therefore, the method supports manufacturing custom-coated drug pellets with defined release properties.
Authors and affiliations
1 IDT Biologika, Am Pharmapark, 06861 Dessau-Roßlau, Germany
2 Department of Applied Biosciences and Process Engineering, Anhalt University of Applied Sciences, Bernburger Straße 55, 06366 Köthen, Germany
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Notations
| M0 | initial drug dose, drug content | (%) |
| Mt | released drug amount at time | (%) |
| AUC | area under the curve | (%*min) |
| DE | dissolution efficiency | (-) |
| MDT | mean dissolution time | (min) |
| Ti | released drug amount at moment t test formulation | (%) |
| Ri | released drug amount at moment t reference formulation | (%) |
| n | number of time points | (-) |
| I | release time point | (min) |
| T | moment of drug release | (min) |
| k0 | release rate constant, zero order release kinetics | (%/min) |
| k1 | release rate constant, first order release kinetics | (1/min) |
| kq | release rate constant, square root release kinetics | (-) |
| kc | release rate constant, cubic root release kinetics | (-) |
| 1/a | scale parameter of Weibull function | (-) |
| b | shape parameter of Weibull function | (-) |
| x50.3 | median of volume density distribution | (µm) |
| R2 | coefficient of determination (CoD) | (-) |
| A | cross section area | (m2] |
| D | diffusion coefficient | (cm2/s) |
| dn/dt | transport flow | (mol/min) |
| dc/dx | concentration gradient | (mol/l*m) |
| f1 | difference factor | (-) |
| f2 | similarity factor | (-) |
Abbreviations
| CoD | coefficient of determination |
| P1, P2, P3 | coated pellet lots, experimental release |
| P1cal, P2cal, P3cal | coated pellet lots, calculated release |
| Ph.Eur. | European Pharmacopoeia |
| pKa | logarithmic acid dissociation constant |
| PVP | polyvinylpyrrolidone |
| rpm | rotation per minute |
| SB pellets | sodium benzoate coated pellets |
| SFV | spatial filter velocimetry |
References
- Koch, H.P. Die Technik der Dissolutionsbestimmung. Teil 2. Experimentelle Methodik, Auflösungsgesetze, Auswertung und graphische Darstellung der Ergebnisse, Kenngrößen der Dissolution, Korrelation. Pharm. Acta Helv. 1984, 59, 130–139. [Google Scholar]
- Yonezawa, Y.; Kawase, S.; Sasaki, M.; Shinohara, I. Dissolution of solid dosage form. V. New form equations for the non-sink dissolution of a monodisperse system. Chem. Pharm. Bull. 1995, 43, 304–310. [Google Scholar] [CrossRef]
- Pereira de Almeida, L.; Simões, S.; Brito, P.; Portugal, A.; Figueiredo, M. Modeling dissolution of sparingly soluble multisized powders. J. Pharm. Sci. 1997, 86, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Sun, J.; Zhao, N.; Sun, M.; He, Z. Preparation and in vitro/in vivo evaluation of sustained-release venlafaxine hydrochloride pellets. Int. J. Pharm. 2012, 426, 21–28. [Google Scholar] [CrossRef]
- Javadzadeh, Y.; Monajjemzadeh, F.; Safaei, E.; Adibkia, K.H. Release kinetics of sodium diclofenac from controlled release device. Pharm. Ind. 2014, 76, 1786–1793. [Google Scholar]
- Viswanadha, L.S.; Arcot, Y.; Lin, Y.-T.; Akbulut, M.E.S. A comparative investigation of release kinetics of paclitaxel from natural protein and macromolecular nanocarriers in nanoscale drug delivery systems. J. Colloid Interf. Sci. Open 2024, 15, 100120. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Li, B.; Fan, R.; Li, B.; Yin, T.; Rong, L.; Sun, J. Preparation and evaluation of tamsulosin hydrochloride sustained-release pellets modified by two-layered membrane techniques. Asian J. Pharm. Sci. 2015, 10, 31–39. [Google Scholar] [CrossRef]
- Kovacevic, J.; Mladenovic, A.; Djuris, J.; Ibric, S. Evaluation of powder, solution and suspension layering for the preparation of enteric coated pellets. Eur. J. Pharm. Sci. 2016, 85, 84–93. [Google Scholar] [CrossRef]
- Cascone, S. Modeling and comparison of release profiles: Effect of the dissolution method. Eur. J. Pharm. Sci. 2017, 106, 352–361. [Google Scholar] [CrossRef]
- Marucci, M.; Andersson, H.; Hjärtstam, J.; Stevenson, G.; Baderstedt, J.; Stading, M.; Larsson, A.; Corswant, C. New insights on how to adjust the release profile from coated pellets by varying the molecular weight of ethyl cellulose in the coating film. Int. J. Pharm. 2013, 458, 218–223. [Google Scholar] [CrossRef]
- Xu, M.; Liew, C.V.; Heng, P.W.S. Evaluation of the coat quality of sustained release pellets by individual pellet dissolution methodology. Int. J. Pharm. 2015, 478, 318–327. [Google Scholar] [CrossRef]
- Villar López, E.; Luzardo Álvarez, A.; Blanco Méndez, J.; Otero Espinar, F.J. Cellulose-polysaccharide film-coating of cyclodextrin based pellets for controlled drug release. J. Drug Deliv. Sci. Technol. 2017, 42, 273–283. [Google Scholar] [CrossRef]
- Sousa, J.J.; Sousa, M.J.; Moura, M.J.; Podczeck, F.; Newton, J.M. The influence of core materials and film coating on the drug release from coated pellets. Int. J. Pharm. 2002, 233, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Krueger, C.; Thommes, M.; Kleinebudde, P. Influence of storage conditions on properties of MCC II-based pellets with theophylline-monohydrate. Eur. J. Pharm. Biopharm. 2014, 88, 483–491. [Google Scholar] [CrossRef]
- Albanez, R.; Nitz, M.; Pereira Taranto, O. Influence of the type of enteric coating suspension, coating layer and process conditions on dissolution profile and stability of coated pellets of diclofenac sodium. Powder Technol. 2015, 269, 185–192. [Google Scholar] [CrossRef]
- Kazlauske, J.; Cafaro, M.M.; Caccavo, D.; Marucci, M.; Lasson, A. Determination of the release mechanism of theophylline from pellets coated with Surelease®—A water dispersion of ethyl cellulose. Int. J. Pharm. 2017, 528, 345–353. [Google Scholar] [CrossRef]
- Hiew, T.N.; Siew, L.W.; Wannaphatchaiyong, S.; Elsergany, R.N.; Pichayakorn, W.; Boonme, P.; Heng, P.W.S.; Liew, C.V. Influence of talc and hydrogenated castor oil on the dissolution behavior of metformin-loaded pellets with acrylic-based sustained release coating. Int. J. Pharm. 2023, 640, 122984. [Google Scholar] [CrossRef]
- Alagili, M.F.; AlQuadeib, B.T.; Ashri, L.Y.; Ibrahim, M.A. Optimization and evaluation of Lisinopril mucoadhesive sustained release matrix pellets: In-vitro and ex-vivo studies. Saudi Pharm. J. 2023, 31, 101690. [Google Scholar] [CrossRef]
- Wu, S.; Zeng, Q.; Zhang, Z.; Zhang, X.; Hou, Y.; Li, Z.; Jia, C.; Liu, Y.; Li, W. Development of sinomenine hydrochloride sustained-release pellet using a novel whirlwind fluidized bed. J. Drug Deliv. Sci. Technol. 2022, 78, 103956. [Google Scholar] [CrossRef]
- Muschert, S.; Siepmann, F.; Leclercq, B.; Carlin, B.; Siepmann, J. Prediction of drug release from ethylcellulose coated pellets. J. Control Release 2009, 135, 71–79. [Google Scholar] [CrossRef]
- Yang, M.; Xie, S.; Li, Q.; Wang, Y.; Chang, X.; Shan, L.; Sun, L.; Huang, X.; Gao, C. Effects of polyvinylpyrrolidone both as a binder and pore-former on the release of sparingly water-soluble topiramate from ethylcellulose coated pellets. Int. J. Pharm. 2014, 465, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Dekyndt, B.; Verin, J.; Neut, C.; Siepmann, F.; Siepmann, J. How to easily provide zero order release of freely soluble drugs from coated pellets. Int. J. Pharm. 2015, 478, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Thapa, R.; Choi, D.H.; Jeong, S.H. Effect of pharmaceutical processes of the quality of ethylcellulose coated pellets: Quality by Design approach. Powder Technol. 2018, 339, 25–38. [Google Scholar] [CrossRef]
- Kaur, S.; Sivasankaran, S.; Wambolt, E.; Jonnalagadda, S. Determinants of zero-order release kinetics from acetaminophen-layered Suglet® pellets, Wurster-coated with plasticized Aquacoat® ECD (ethyl cellulose dispersion). Int. J. Pharm. 2020, 573, 118873. [Google Scholar] [CrossRef]
- Kállai, N.; Luhn, O.; Dredán, J.; Kovács, K.; Lengyel, M.; Antal, I. Evaluation of drug release from coated pellets based on isomalt, sugar and microcrystalline cellulose inert cores. AAPS PharmSciTech 2010, 11, 383–391. [Google Scholar] [CrossRef]
- Priese, F.; Wolf, B. Development of high drug loaded pellets by Design of Experiment and population balance model calculation. Powder Technol. 2013, 241, 149–157. [Google Scholar] [CrossRef]
- Wiegel, D.; Eckardt, G.; Priese, F.; Wolf, B. In-line particle size measurement and agglomeration detection of pellet fluidized bed coating by Spatial Filter Velocimetry. Powder Technol. 2016, 301, 261–267. [Google Scholar] [CrossRef]
- Petrak, D.; Eckardt, G.; Dietrich, S.; Köhler, M.; Wiegel, D.; Wolf, B.; Priese, F.; Jacob, M. In-line measurement of layer thickness, agglomerate fraction and spray drying during pellet coating in the fluidized bed. Pharm. Ind. 2018, 80, 262–270. [Google Scholar]
- Priese, F.; Frisch, T.; Wolf, B. Comparison of film-coated retarded release pellets manufactured by layering technique or by fluidized bed rotor pelletization. Pharm. Dev. Technol. 2015, 20, 417–424. [Google Scholar] [CrossRef]
- Juhász, A.; Ungor, D.; Berta, K.; Seres, L.; Csapó, E. Spreadsheet-based nonlinear analysis of in vitro release properties of a model drug from colloidal carriers. J. Mol. Liq. 2021, 328, 115405. [Google Scholar] [CrossRef]
- European Pharmacopoeia 11.5, 2024. Available online: https://pheur.edqm.eu/subhome/11-5 (accessed on 29 August 2024).