Introduction
The development of a hydroxynorketamine modified-release dosage form marks an important advance in neuropsychiatric therapy. Hydroxynorketamine (HNK), a ketamine metabolite, shows rapid antidepressant activity through mechanisms different from ketamine itself. It works mainly by modulating α7-nicotinic acetylcholine receptors and activating mTOR pathways.
This targeted action makes HNK a strong candidate as an active pharmaceutical ingredient with a favorable safety profile. Unlike ketamine, it avoids dissociative and addictive side effects. A modified-release form built with CELLETS®—uniform spherical pellets—offers tighter therapeutic control. It sustains plasma concentration, reduces peak-to-trough swings, and helps patients stay consistent with treatment.
In addition, the inert cores often range between 100 and 500 μm in size. A more refined range of 200 to 400 μm improves precision. About 90% of particles fall within this window, confirmed by sieve analysis. One example is CELLETS® 200, which demonstrates this particle size distribution effectively.
API Function and Patient Benefits
Hydroxynorketamine mainly acts by inhibiting α7-nicotinic receptors. This lowers intracellular Ca²⁺ and D-serine levels and reduces NMDA receptor excitotoxicity. At the same time, it boosts mTOR signaling and strengthens AMPA receptor function.
Together, these effects speed up synaptogenesis and create fast antidepressant responses. Evidence comes from both preclinical studies and early clinical findings. For patients, this means rapid mood elevation without ketamine-related side effects. Unlike ketamine, it does not cause hallucinations or carry strong abuse potential.
From a pharmacokinetic view, a modified-release dosage form improves consistency in therapy. It also simplifies dosing schedules and increases tolerability.
Modified‑release dosage Formulation with CELLETS®
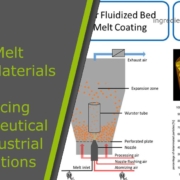
The incorporation of CELLETS® into the modified‑release formulation provides several benefits. Their uniform size and high sphericity ensure consistent drug coating and predictable release. CELLETS® also enable multiparticulate dosing, which reduces variability and allows tailored release profiles.
For hydroxynorketamine (HNK), CELLETS® can carry specific polymer coatings such as ethylcellulose or Eudragit. These coatings dissolve or erode at controlled rates, releasing the API steadily over time. This method lowers peak systemic concentrations, which reduces side effects while maintaining efficacy.
Additionally, CELLETS® support monolithic layering or reservoir systems. This setup allows complex release patterns, such as an initial burst followed by sustained delivery. Such profiles are ideal for achieving a rapid onset and maintaining antidepressant effects in depression treatment.
Key Findings on Hydroxynorketamine modified‑release dosage form
In the disclosed patent (US 2025 0177325 A1), researchers describe a multiparticulate modified‑release system for hydroxynorketamine. They use CELLETS® as the core substrate. The CELLETS® carry successive polymer layers that control drug release. This design produces an initial release phase followed by prolonged delivery.
Pharmacokinetic modeling shows a flattened plasma-concentration profile, lower maximum concentration (Cmax), longer time to peak (Tmax), and higher area under the curve (AUC). Together, these factors maintain therapeutic HNK levels over time. This steady exposure may reduce rebound symptoms and cut dosing frequency. As a result, patient adherence improves, and treatment regimens may shift to once-daily or even less frequent dosing.
Conclusion and Outlook
In conclusion, the hydroxynorketamine modified‑release dosage form using CELLETS® offers a promising pharmaceutical approach. It leverages HNK’s unique mechanism as a non-dissociative antidepressant. Controlled release maximizes its clinical potential.
Cellet-based formulations improve pharmacokinetics, enhance tolerability, and increase convenience. These benefits could significantly help patients with treatment-resistant depression. Further work is needed, including in vitro−in vivo correlation studies, polymer selection optimization, and confirmatory clinical trials.
Looking ahead, this technology may expand HNK applications to other neuropsychiatric or neurodegenerative disorders. It provides a refined dosage form that meets both patient needs and therapeutic goals.
Patent Details
- Name or patent: Hydroxynorketamine for the use in the treatment of depression
- Patent number: US 20250177325 A1
- Year of patent: 2025
- Patent holder names and affiliation: (Names not specified in public abstract; likely the inventors assigned to their sponsoring institution or company as listed in patent document)
This summary underscores the innovative use of CELLETS® in creating a refined hydroxynorketamine modified-release dosage form that elevates both therapeutic performance and patient-centric outcomes.







 adobe firefly
adobe firefly 
