Colon delivery of vitamin B2
This article “In vitro validation of colon delivery of vitamin B2 through a food grade multi-unit particle system” discusses a novel method for delivering active ingredients, particularly riboflavin, to the colon in a food-grade, environmentally friendly form using a double-layer coated multi-unit particle system (MUPS). The MUPS uses a shellac outer layer, alginate inner layer, and cellulose core, maintaining integrity through upper digestive processes. Tests showed it releases about 90% of riboflavin in the colon, enhancing gut health by promoting beneficial short-chain fatty acids. This sustainable approach addresses rising demand for effective colon-targeted health products and aligns with EU regulations limiting microplastic use in consumable goods.
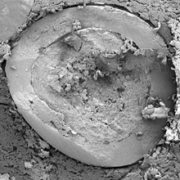
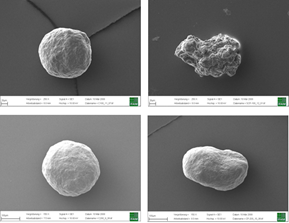
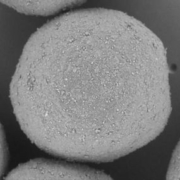
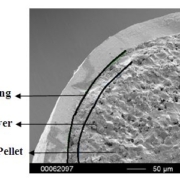
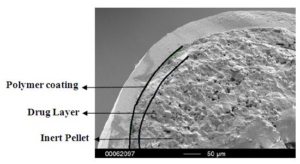
The MUPS containing riboflavin, branded as Humiome® B2 by DSM-Firmenich, utilizes cellulose pellets known as CELLETS® as the core material. The manufacturing process involves a fluid bed layering method, where riboflavin and pectin are applied as a binder onto the Cellets. The MUPS is then coated with layers of sodium alginate and hardened with calcium chloride, followed by a shellac outer layer. This design ensures a controlled, colonic release, offering an efficient, food-grade delivery system for active nutrients.
The study demonstrates the efficacy of a shellac-alginate MUPS for targeted delivery of riboflavin to the colon, using food-grade materials that align with environmental standards. In vitro models validated its effectiveness, with around 90% of riboflavin reaching the colonic region. Results show promise for health benefits linked to microbiome modulation and short-chain fatty acid production. Future clinical studies will focus on the impact of this delivery system on microbiome and host health, supporting its potential in functional foods, supplements, and medical nutrition.
Abstract
Colon target delivery of active ingredients is frequently applied in pharmaceutical products. However, in functional food and beverage applications, dietary supplements, and medical nutrition, formats targeting colonic delivery to improve human health are rare. Nevertheless, there is emerging evidence for beneficial effects of colonic delivered nutrients on gut microbiota and host health which increases the demand for sustainable food grade materials that are regulatory approved for application. In this paper, we describe a double layer coated multi-unit particle system (MUPS) with a diameter of approximately 730 microns consisting of food grade materials: shellac as outer layer, alginate as inner layer, cellulose as a core and riboflavin as active ingredient. The suitability of the MUPS for colonic delivery was tested in three well-established in vitro digestion and fermentation models: the USP Apparatus 3 and the TNO Intestinal Models 1 and 2 (TIM-1 and TIM-2). All systems confirmed the integrity of the MUPS under simulated upper gastrointestinal tract conditions with approximately 90% of the active ingredient being released under simulated ileal-colonic conditions. The TIM-2 model also showed the effects of riboflavin loaded MUPS on the microbiome composition with an increase in the production of short-chain fatty acids, acetate and butyrate. The results of these experiments provide a reliable basis for validation of this vitamin-loaded food grade MUPS in future human clinical trials. In addition, following the recent announcement of the European Commission to restrict intentionally added microplastics to products, the materials used in the described formulation offer an environmentally friendly alternative to often applied methyl acrylate based coatings.
Source of Abstract


 ingredientpharm
ingredientpharm glatt.com
glatt.com