Microcrystalline Cellulose (MCC) is made from wood pulp. It is used in pharmaceutical formulations as excipients. MCC can be pelletized as forms inert highly spherical starter beads (such as CELLETS®).
Posts
What is a nutritional table for CELLETS® about? CELLETS® consist of 100% microcrystalline cellulose (MCC) and were developed for advanced oral dosage forms, such as MUPS, capsules, sachets, and stick pack units. Some pharmaceutical applications now overlap with food products and vice versa. The nutraceutical market is growing, and we frequently receive questions about the energy and nutritional content of MCC. Let’s clarify this.
MCC is a modified form of cellulose commonly used as a filler and stabilizer in the food and pharmaceutical industries. It consists of plant fibers and acts mainly as a dietary fiber. It contains almost no usable nutrients, such as vitamins, minerals, proteins, or fats. Since the human body cannot digest MCC, it provides no calories.
Because the body does not metabolize microcrystalline cellulose, it does not supply any macro- or micronutrients. Therefore, a nutritional table for 100 g of MCC would look like this:
| Substance | quantity per 100 g |
|---|---|
| Energy | 0 kcal |
| Protein | 0 g |
| Fat | 0 g |
| Carbohydrates | 0 g |
| Dietary fiber | 100 g |
| Sugar | 0 g |
| Salt | 0 g |
| Vitamins & minerals | 0 mg |
Microcrystalline cellulose is a pure dietary fiber with no nutritional value. The human digestive tract does not break it down or absorb it [1]. As a result, it provides no calories and no nutrients to the body. The same nutritional profile applies to CELLETS®, although it rarely matters in pharmaceutical formulations.
References
[1] N. Prabsangob, NFS Journal, 31 (2023) 39-49. doi:10.1016/j.nfs.2023.03.002
Abstract
References to “The Increase in the Plasticity of Microcrystalline Cellulose Spheres’ When Loaded with a Plasticizer”
Authors: Artūrs Paulausks, Tetiana Kolisnyk and Valentyn Mohylyuk
First published: Pharmaceutics 2024, 16(7), 945; https://doi.org/10.3390/pharmaceutics16070945
1. Introduction
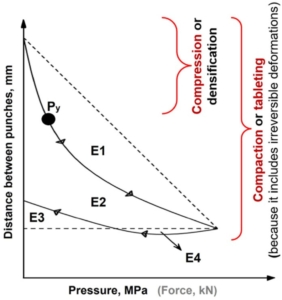
Figure 1. An example of a force-displacement profile highlighting: the rearrangement energy (E1), plastic energy (E2 + E4), elastic energy (E3; or energy lost), plastic flow energy (E4), compaction energy (E1 + E2 + E3), and mean yield pressure (Py). The arrows on the curve are showing the direction of curve development.
2. Materials and Methods
2.1. Materials
2.2. Theoretical Solubility Parameter Computation
The approach authored by Van Krevelen and Hoftyzer estimates a high likelihood of successful mixing of two substances if the parameter ΔδT (Equation (1)) is not more than 5 MPa0.5, while complete immiscibility occurs when ΔδT exceeds 10 MPa0.5 [30,31].
By Bagley’s approach, the drug–polymer miscibility is evaluated using the combined solubility parameter δv (Equation (2)).
The probability of miscibility is concluded if the distance between two points in the two-dimensional plot is D12 ≤ 5.0 (Equation (3)) [31].
The approach by Greenhalgh evaluates the miscibility as the absolute difference Δδt (Equation (4)) between the total solubility parameters δt which are calculated from Equation (5).
2.3. Plasticizer Loading onto MCC Cores Using Solvent Evaporation Method
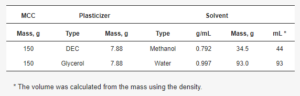
Table 1. Used amounts of plasticizer and solvent for the plasticizer loading procedure.
2.4. Thermogravimetric Analysis (TGA)
2.5. Differential Scanning Calorimetry (DSC)
2.6. Powder X-ray Diffraction (pXRD) Analysis
2.7. Fourier-Transform Infrared (FTIR) Attenuated Total Reflectance (ATR) Spectroscopy
2.8. Scanning Electron Microscopy (SEM) and Particle Size Distribution Analysis
2.9. Preparation of Tablets
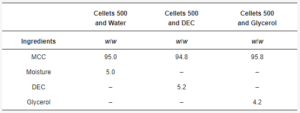
Table 2. Formulations for tableting.
2.10. The Theoretical True Density Calculation
The theoretical true density of tablet composition was calculated based on the pycnometric density (ρt) of MCC (1.586 g/cm3) [16,33], glycerol (1.262 g/cm3) [34], DEC (1.287 g/cm3) [35], and their shares (x, w/w) using the additive methodology and the following equation [1]:
2.11. In-Die Heckel Plot Construction
The relative density (ln(1/ε)) was calculated automatically with Alix software ver. 20220711 (Medelpharm, Beynost, France) [4]. The relative density and compaction pressure (P, MPa) data were plotted by the Heckel relationship [6]:
where: K is the slope of the linear region (the proportionality constant), and ln(1/ε0) is a constant, A, that represents the intercept/ degree of packing (at porosity ε0) achieved at low pressure because of the rearrangement process before an appreciable amount of interparticle bonding takes place. The mean yield pressure (Py, MPa) was calculated in accordance with Hersey and Rees by the equation [3,7,36]:
3. Results and Discussion

Figure 2. Evaluation of MCC–plasticizer miscibility using averaged solubility parameters: 3D approach authored by Hoftyzer and Van Krevelen (a), 2D Bagley’s plot (b), and 1D bar graph according to Greenhalgh (c).
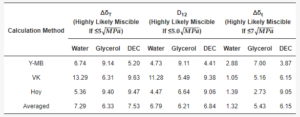
Table 3. Hansen solubility parameter calculations.

Figure 3. SEM of MCC spheres (D10% = 563 µm, D50% = 651 µm, and D90% = 696 µm).
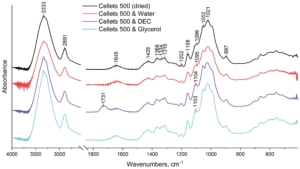
Figure 4. FTIR spectrum of dried and loaded MCC spheres in the range of 4000–500 cm−1.
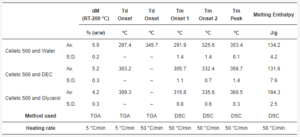
Table 4. The summary of thermal properties determined by TGA and DSC.
-
The broad peak at 3333 cm−1 which is assigned to O–H stretching vibrations of the intermolecularly bonded hydroxyl group;
-
The peak at 2891 cm−1 that corresponds to C–H stretching vibrations;
-
The peak at 1645 cm−1 which is indicative of the O–H bending of bound water;
-
The multiple absorbance bands (peaks at 1428, 1368, 1334, and 1316 cm−1) assigned to the bending and stretching vibrations of C–H and C–O bonds;
-
The peaks at 1202, 1052, and 1021 cm−1 are assigned to the elongation of C-O bonds;
-
The peaks at 1158 and 897 cm−1 are due to the C–O–C stretching vibrations at the β-glycosidic linkage.
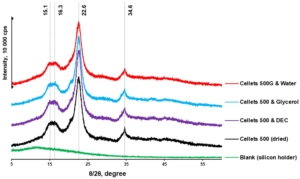
Figure 5. pXRD diffractograms of dried and loaded MCC spheres.
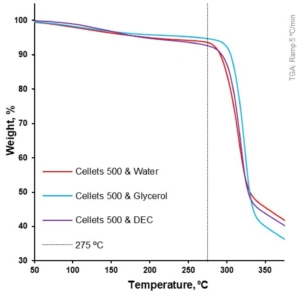
Figure 6. Weight loss as a function of temperature for loaded MCC spheres (TGA at 5 °C/min).
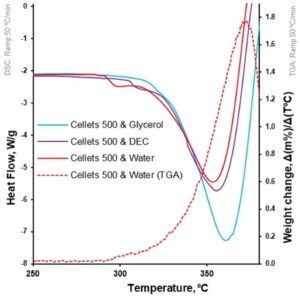
Figure 7. Heat flow as function of temperature for loaded MCC spheres (DSC at 50 °C/min; left Y-axis); the first derivative of weight loss as a function of temperature for water-loaded MCC spheres (TGA at 50 °C/min; right Y-axis).
Tableting of plasticizer-loaded MCC spheres with a compaction simulator was illustrated with pressure-displacement profiles (Figure 8a; exemplified with glycerol-loaded MCC spheres), which were converted to in-die Heckel plots (Figure 8b).
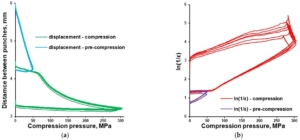
Figure 8. In-die Heckel plot (a) and pressure-displacement profile (b) for MCC spheres (CELLETS® 500) loaded with glycerol.
The mean yield pressure (Py) of non-plasticized MCC was found at the level of 136.5 ± 6.9 MPa (Av. ± S.D.). Despite the sequence of Tm onsets, the mean yield pressure of plasticizer-loaded MCC spheres decreased from DEC (124.7 ± 9.2 MPa) to water (106.6 ± 10.0 MPa) and glycerol (99.9 ± 1.9 MPa; Figure 9, Table 5). That coincided with the miscibility likelihood order based on the HSP calculations. Therefore DEC, water, and glycerol were able to decrease the Py of non-plasticized MCC spheres by 4.7, 16.3, and 38.9%, respectively.
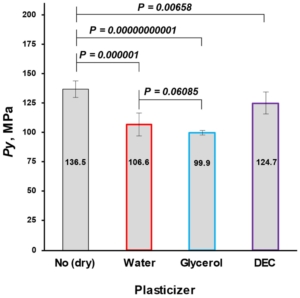
Figure 9. Comparison of non-plasticized (dried) with plasticized MCC spheres (Cellets® 500) in terms of Py (a plasticity parameter).
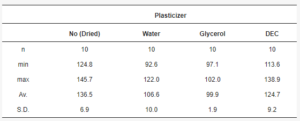
Table 5. Py data statistics.
4. Conclusions
Additonal information on: Plasticity of Microcrystalline Cellulose Spheres
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Lisence
References
- Mohylyuk, V.; Bandere, D. High-speed tableting of high drug-loaded tablets prepared from fluid-bed granulated isoniazid. Pharmaceutics 2023, 15, 1236. [Google Scholar] [CrossRef] [PubMed]
- Romerova, S.; Dammer, O.; Zamostny, P. Development of an Image-based Method for Tablet Microstructure Description and Its Correlation with API Release Rate. AAPS PharmSciTech 2023, 24, 199. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, N.A. Tablet Manufacture. In Encyclopedia of Pharmaceutical Technology; Swabrick, J., Ed.; Informa Healthcare USA, Inc.: New York, NY, USA, 2007; pp. 3653–3672. [Google Scholar]
- User Guide and Reference Manual of Software Alix (PR-W3-002); Korsch/MEDELPHARM: Berlin, Germany, 2020.
- Tay, J.Y.S.; Kok, B.W.T.; Liew, C.V.; Heng, P.W.S. Effects of Particle Surface Roughness on In-Die Flow and Tableting Behavior of Lactose. J. Pharm. Sci. 2019, 108, 3011–3019. [Google Scholar] [CrossRef] [PubMed]
- Heckel, R.W. Density-pressure relationships in powder compaction. Trans. Metal. Soc. AIME 1961, 221, 671–675. [Google Scholar]
- Hersey, J.A.; Rees, J.E. Deformation of Particles during Briquetting. Nat. Phys. Sci. 1971, 230, 96. [Google Scholar] [CrossRef]
- Vreeman, G.; Sun, C.C. Mean yield pressure from the in-die Heckel analysis is a reliable plasticity parameter. Int. J. Pharm. X 2021, 3, 100094. [Google Scholar] [CrossRef] [PubMed]
- Mohylyuk, V.; Paulausks, A.; Radzins, O.; Lauberte, L. The Effect of Microcrystalline Cellulose–CaHPO4 Mixtures in Different Volume Ratios on the Compaction and Structural–Mechanical Properties of Tablets. Pharmaceutics 2024, 16, 362. [Google Scholar] [CrossRef] [PubMed]
- Mohylyuk, V. Effect of roll compaction pressure on the properties of high drug-loaded piracetam granules and tablets. Drug Dev. Ind. Pharm. 2022, 48, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, J.S.; Hong, S.; Ha, E.S.; Nie, H.; Zhou, Q.T.; Kim, M.S. Tableting process-induced solid-state polymorphic transition. J. Pharm. Investig. 2022, 52, 175–194. [Google Scholar] [CrossRef]
- Thio, D.R.; Heng, P.W.S.; Chan, L.W. MUPS Tableting—Comparison between Crospovidone and Microcrystalline Cellulose Core Pellets. Pharmaceutics 2022, 14, 2812. [Google Scholar] [CrossRef] [PubMed]
- Dashevsky, A.; Kolter, K.; Bodmeier, R. Compression of pellets coated with various aqueous polymer dispersions. Int. J. Pharm. 2004, 279, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Plumpton, E.J.; Gilbert, P.; Fell, J.T. The survival of microorganisms during tabletting. Int. J. Pharm. 1986, 30, 241–246. [Google Scholar] [CrossRef]
- Vorlander, K.; Bahlmann, L.; Kwade, A.; Finke, J.H.; Kampen, I. Effect of Process Parameters, Protectants and Carrier Materials on the Survival of Yeast Cells during Fluidized Bed Granulation for Tableting. Pharmaceutics 2023, 15, 884. [Google Scholar] [CrossRef] [PubMed]
- Galichet, L.Y. Cellulose Microcrystalline. In Handbook of Pharmaceutical Excipients; Rowe, R.C., Sheskey, P.J., Owen, S.C., Eds.; Pharmaceutical Press: London, UK; American Pharmacists Association: Grayslake, IL, USA, 2006. [Google Scholar]
- de la Luz Reus Medina, M.; Kumar, V. Comparative evaluation of powder and tableting properties of low and high degree of polymerization cellulose I and cellulose II excipients. Int. J. Pharm. 2007, 337, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.C. Mechanism of moisture induced variations in true density and compaction properties of microcrystalline cellulose. Int. J. Pharm. 2008, 346, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Chamarthy, S.P.; Diringer, F.X.; Pinal, R. The Plasticization-Antiplasticization Threshold of Water in Microcrystalline Cellulose: A Perspective Based on Bulk Free Volume. In Water Properties in Food, Health, Pharmaceutical and Biological Systems; Reid, D.S., Sajjaanantakul, T., Eds.; Wiley-Blackwell: Hoboken, NJ, USA; Singapore, 2010; pp. 301–314. [Google Scholar]
- Osei-Yeboah, F. Improving Powder Tableting Performance through Materials Engineering. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 2015. [Google Scholar]
- Siepmann, F.; Siepmann, J.; Walther, M.; MacRae, R.; Bodmeier, R. Aqueous HPMCAS coatings: Effects of formulation and processing parameters on drug release and mass transport mechanisms. Eur. J. Pharm. Biopharm. 2006, 63, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, M.; Bodmeier, R. Influence of plasticization time, curing conditions, storage time, and core properties on the drug release from Aquacoat-coated pellets. Pharm. Dev. Technol. 2001, 6, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Matic, J.; Paudel, A.; Bauer, H.; Garcia, R.A.L.; Biedrzycka, K.; Khinast, J.G. Developing HME-Based Drug Products Using Emerging Science: A Fast-Track Roadmap from Concept to Clinical Batch. AAPS PharmSciTech 2020, 21, 176. [Google Scholar] [CrossRef] [PubMed]
- Oladeji, S.; Mohylyuk, V.; Jones, D.S.; Andrews, G.P. 3D printing of pharmaceutical oral solid dosage forms by fused deposition: The enhancement of printability using plasticised HPMCAS. Int. J. Pharm. 2022, 616, 121553. [Google Scholar] [CrossRef]
- Klar, F.; Urbanetz, N.A. Solubility parameters of hypromellose acetate succinate and plasticization in dry coating procedures. Drug Dev. Ind. Pharm. 2016, 42, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- Medarevic, D.; Djuriš, J.; Barmpalexis, P.; Kachrimanis, K.; Ibrić, S. Analytical and Computational Methods for the Estimation of Drug-Polymer Solubility and Miscibility in Solid Dispersions Development. Pharmaceutics 2019, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Kitak, T.; Dumičić, A.; Planinšek, O.; Šibanc, R.; Srčič, S. Determination of Solubility Parameters of Ibuprofen and Ibuprofen Lysinate. Molecules 2015, 20, 21549–21568. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.M. Hansen. Solubility Parameters: A User’s Handbook; CRC Press LLC: Boca Raton, FL, USA, 2000. [Google Scholar]
- Kolisnyk, T.; Mohylyuk, V.; Andrews, G.P. Drug-Polymer Miscibility and Interaction Study as a Preliminary Step in Amorphous Solid Dispersion Development: Comparison of Theoretical and Experimental Data. Maced. Pharm. Bull. 2023, 69, 59–60. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Nijenhuis, K.T. Cohesive Properties and Solubility. In Properties of Polymers; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Jankovic, S.; Tsakiridou, G.; Ditzinger, F.; Koehl, N.J.; Price, D.J.; Ilie, A.R.; Kalantzi, L.; Kimpe, K.; Holm, R.; Nair, A.; et al. Application of the solubility parameter concept to assist with oral delivery of poorly water-soluble drugs—A PEARRL review. J. Pharm. Pharmacol. 2019, 71, 441–463. [Google Scholar] [CrossRef] [PubMed]
- Pitzanti, G.; Mohylyuk, V.; Corduas, F.; Byrne, N.M.; Coulter, J.A.; Lamprou, D.A. Urethane dimethacrylate-based photopolymerizable resins for stereolithography 3D printing: A physicochemical characterisation and biocompatibility evaluation. Drug Deliv. Transl. Res. 2023, 14, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Elsergany, R.N.; Vreeman, G.; Sun, C.C. An approach for predicting the true density of powders based on in-die compression data. Int. J. Pharm. 2023, 637, 122875. [Google Scholar] [CrossRef] [PubMed]
- Price, J.C. Glycerin. In Handbook of Pharmaceutical Excipients; Rowe, R.C., Sheskey, P.J., Owen, S.C., Eds.; Pharmaceutical Press: London, UK; American Pharmacists Association: Grayslake, IL, USA, 2006. [Google Scholar]
- CAS Data Base: DIETHYL CITRATE; ChemicalBook: San Jose, CA, USA, 2023.
- Fell, J.T.; Newton, J.M. Effect of particle size and speed of compaction on density changes in tablets of crystalline and spray-dried lactose. J. Pharm. Sci. 1971, 60, 1866–1869. [Google Scholar] [CrossRef] [PubMed]
- Panaitescu, D.M.; Vizireanu, S.; Stoian, S.A.; Nicolae, C.A.; Gabor, A.R.; Damian, C.M.; Trusca, R.; Carpen, L.G.; Dinescu, G. Poly(3-hydroxybutyrate) Modified by Plasma and TEMPO-Oxidized Celluloses. Polymers 2020, 12, 1510. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Chen, L. Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J. Mater. Chem. A 2013, 1, 3938–3944. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.L. Preparation and properties of cellulose nanocrystals: Rods, spheres, and network. Carbohydr. Polym. 2010, 82, 329–336. [Google Scholar] [CrossRef]
- Agrebi, F.; Ghorbel, N.; Bresson, S.; Abbas, O.; Kallel, A. Study of nanocomposites based on cellulose nanoparticles and natural rubber latex by ATR/FTIR spectroscopy: The impact of reinforcement. Polym. Compos. 2018, 40, 2076–2087. [Google Scholar] [CrossRef]
- Li, M.; He, B.; Chen, Y.; Zhao, L. Physicochemical Properties of Nanocellulose Isolated from Cotton Stalk Waste. ACS Omega 2021, 6, 25162–25169. [Google Scholar] [CrossRef] [PubMed]
- Haafiz, M.K.; Xue, J.F.; Xu, M.; Gui, B.S.; Kuang, L.; Ouyang, J.M. Isolation and characterization of cellulose nanowhiskers from oil palm biomass microcrystalline cellulose. Carbohydr. Polym. 2014, 103, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Xue, J.F.; Xu, M.; Gui, B.S.; Kuang, L.; Ouyang, J.M. Coordination dynamics and coordination mechanism of a new type of anticoagulant diethyl citrate with Ca2+ ions. Bioinorg. Chem. Appl. 2013, 2013, 354736. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.D.; Flores, S.K.; Marangoni, A.G.; Gerschenson, L.N.; Rojas, A.M. Development of a high methoxyl pectin edible film for retention of l-(+)-ascorbic acid. J. Agric. Food Chem. 2009, 57, 6844–6855. [Google Scholar] [CrossRef] [PubMed]
- Armylisas, A.H.N.; Hoong, S.S.; Ismail, T.N.M.T. Characterization of crude glycerol and glycerol pitch from palm-based residual biomass. Biomass Conv. Bioref. 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cielecka, I.; Szustak, M.; Kalinowska, H.; Gendaszewska-Darmach, E.; Ryngajłło, M.; Maniukiewicz, W.; Bielecki, S. Glycerol-plasticized bacterial nanocellulose-based composites with enhanced flexibility and liquid sorption capacity. Cellulose 2019, 26, 5409–5426. [Google Scholar] [CrossRef]
- Turner, A.H.; Holbrey, J.D. Investigation of glycerol hydrogen-bonding networks in choline chloride/glycerol eutectic-forming liquids using neutron diffraction. Phys. Chem. Chem. Phys. 2019, 21, 21782–21789. [Google Scholar] [CrossRef] [PubMed]
- Mohylyuk, V.; Pauly, T.; Dobrovolnyi, O.; Scott, N.; Jones, D.S.; Andrews, G.P. Effect of carrier type and Tween® 80 concentration on the release of silymarin from amorphous solid dispersions. J. Drug Deliv. Sci. Technol. 2021, 63, 102416. [Google Scholar] [CrossRef]
- Song, M.; Liebenberg, W.; De Villiers, M.M. Comparison of high sensitivity micro differential scanning calorimetry with X-ray powder diffractometry and FTIR spectroscopy for the characterization of pharmaceutically relevant non-crystalline materials. Die Pharm.-Int. J. Pharm. Sci. 2006, 61, 336–340. [Google Scholar]
- Dedroog, S.; Pas, T.; Vergauwen, B.; Huygens, C.; Van den Mooter, G. Solid-state analysis of amorphous solid dispersions: Why DSC and XRPD may not be regarded as stand-alone techniques. J. Pharm. Biomed. Anal. 2020, 178, 112937. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yin, L.; Gray, D.L.; Thomas, L.C.; Schmidt, S.J. Impact of sucrose crystal composition and chemistry on its thermal behavior. J. Food Eng. 2017, 214, 193–208. [Google Scholar] [CrossRef]
- Tong, Q.; Xiao, Q.; Lim, L.T. Effects of glycerol, sorbitol, xylitol and fructose plasticisers on mechanical and moisture barrier properties of pullulan–alginate–carboxymethylcellulose blend films. Int. J. Food Sci. Technol. 2012, 48, 870–878. [Google Scholar] [CrossRef]
- Amidon, G.E.; Houghton, M.E. The effect of moisture on the mechanical and powder flow properties of microcrystalline cellulose. Pharm. Res. 1995, 12, 923–929. [Google Scholar] [CrossRef] [PubMed]
Abstract
Microcrystalline Cellulose (MCC) pellets represent a chemically inert class of active pharmaceutical ingredients (API) carriers. A narrow particle size distribution (PSD) maximizes control over content uniformity. In this case study, we will focus on measuring the particle size distribution and on sphericity.
Pellets for oral drug forms
MCC pellets are used as starter beads for API loading. Low or high drug dose loading is technically feasible. These pellets are made of pure MCC and provide a robust platform for delivery of one or multiple APIs. Certain processing technologies for pellet coating allow these starter beads to be compatible with soluble or insoluble APIs, e.g. by Wurster bottom spray [1,2] or Rotor dry powder layering technology [3]. Coated pellets can be filled into capsules, or compacted into multiple-unit pellet system (MUPS) tablets [4], where a tight PSD maximizes control over content uniformity.
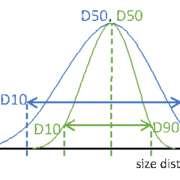
Particle size distribution maximizes the control over content uniformity in applications of complex oral dosing forms. Speaking about uniform or monodisperse particles, these information always point to general information of the particular system, not of the individual particle itself. Therefore, PSD is a globular measure allowing simple, easy and fast analysis of the particulate matter. Major key information from a PSD measure are the so called D-values. A Dx value represents a dimension, where a ratio of X particles is smaller. For reasons of simplicity weighted functions, such as number, radius or volume, are not. Extending these metrics to D10 and D90 additionally informs about the width of the entire size distribution (Figure 1).
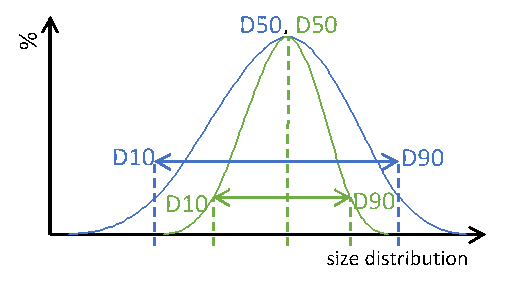
Particle size distribution of two different particle systems with identical median dimension D50. Blue: wide PSD, green: narrow PSD. Dotted lines are guides to the eyes.
Figure 1: Particle size distribution of two different particle systems with identical median dimension D50. Blue: wide PSD, green: narrow PSD. Dotted lines are guides to the eyes.
Dimensions of pellets
In this study, imaging technology (Horiba, Camsizer) was employed for the size analysis. Representatively, more than 50 charges of Cellets® 100 and Cellets® 500 (Figures 2-3) have been analyzed for the D10, D50 and D90 values.
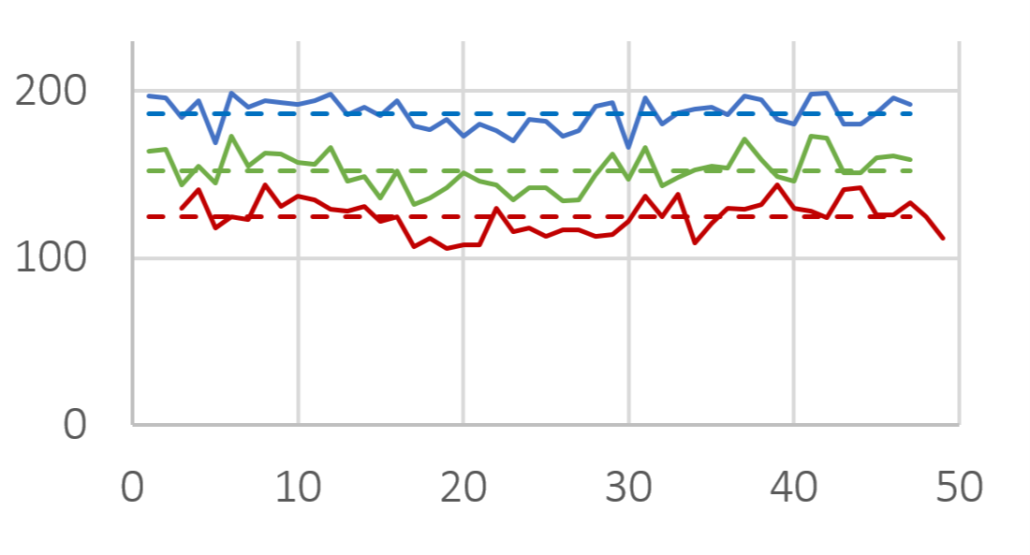
D10 (red), D50 (green) and D90 (blue) value for several Cellets® 100 charges. Solid lines are measures, dashed lines represent the averaged value of all charges. The standard deviation is below 10 %.
Figure 2: D10 (red), D50 (green) and D90 (blue) value for several Cellets® 100 charges. Solid lines are measures, dashed lines represent the averaged value of all charges. The standard deviation is below 10 %.
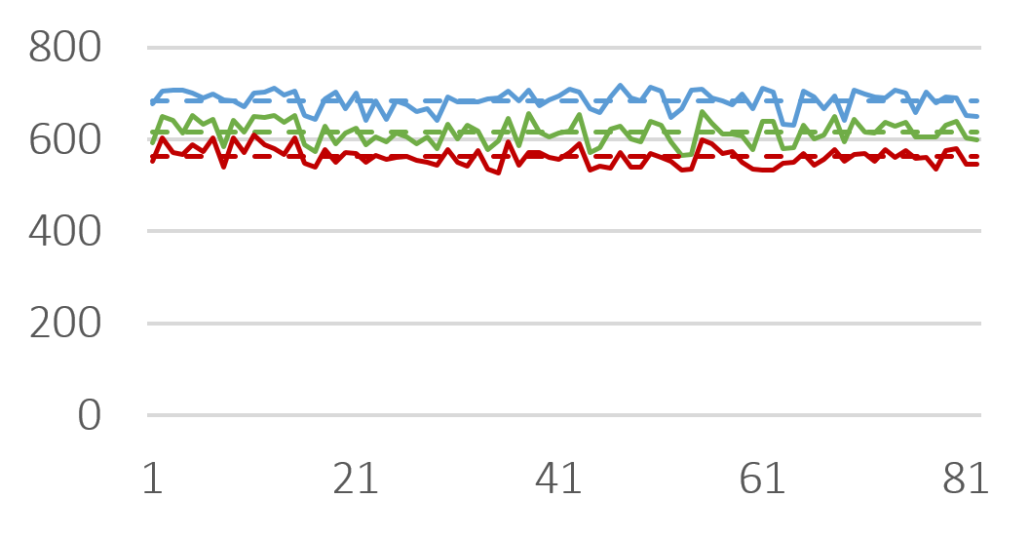
D10 (red), D50 (green) and D90 (blue) value for several Cellets® 500 charges. Solid lines are measures, dashed lines represent the averaged value of all charges. The standard deviation is below 4 %.
Figure 3: D10 (red), D50 (green) and D90 (blue) value for several Cellets® 500 charges. Solid lines are measures, dashed lines represent the averaged value of all charges. The standard deviation is below 4 %.
The results show only slight variations in the PSD between the charges. The standard deviation is smaller than 4 % (Cellets® 500) and smaller than 10 % (Cellets® 100) which confirms a high reproducibility in production (Table 1). Both values are remarkably good for technical spheres. Furthermore, none of the charges was out of specifications and fit into the desired size distribution between 500 µm and 710 µm easily. The close gap between D10 and D90 clearly identify an excellent monodispersity.
| Standard deviation | Cellets 100 | Cellets 500 |
| of D10 | 8.28 % | 3.97 % |
| of D50 | 7.12 % | 3.52 % |
| of D90 | 4.68 % | 3.11 % |
Table 1: Standard deviation for D10, D50 and D90 values Cellets® 100 and Cellets® 500 charges.
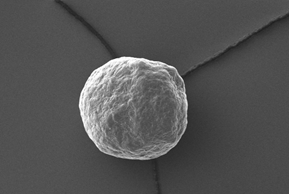
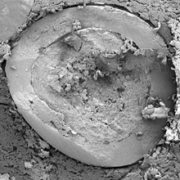
Electron microscopy yield perfect imaging data of the MCC pellets’ surfaces. Magnification: 250x, working distance 8.0 mm, voltage: 10 keV.
Figure 4: Electron microscopy yield perfect imaging data of the MCC pellets’ surfaces. Magnification: 250x, working distance 8.0 mm, voltage: 10 keV.
Perfect sphericity? – Yes!
For a more detailed shape analysis, electron microscopy yield perfect imaging data of the MCC pellets’ surfaces (Figure 4). Additionally, MCC pellets have a distinguishing friability.
Summary
Microcrystalline Cellulose (MCC) pellets show excellent chemically inertness, high degree of sphericity, narrow size distribution and high reproducibility in production. These properties make Cellets® becoming one of the first choice for inert API carriers. We have proven these excellent properties for Cellets® 100 and Cellets® 500. The obtained results are representative for other size classes ranging from 100 µm to 1400 µm.
Acknowledgement
We acknowledge IPC Process-Center (Dresden, Germany) for the analytics, and Fraunhofer IFAM (Dresden, Germany) for recording the electron microscopic pictures.
References
[1] H. R. Norouzi, International Journal of Pharmaceutics, Volume 590 (2020) 119931
[2] D. Jones, Developing Solid Oral Dosage Forms, Pharmaceutical Theory And Practice (2009) 807-825
[4] S. Abdul, A. Chandewar, S. Jaiswal, Journal of Controlled Release, Volume 147(1) (2010) 2-16
Abstract
Several drug substances are known to be extremely bitter. In special for paediatric and geriatric applications, costumer compliance by means of taste acceptance is a highly sensitive topic. Taste masking is therefore an important issue in pharmaceutical industry of oral dosage forms not only to improve the compliance, but it is also used to define a unique taste identification by the costumer (competition advantages). This case study will focus on taste-masking of an API and on the optimization of release in the absorption window of the API.
Modifying the taste
Several methods are available to actively influence, hide or modify taste, such as the initial activation of the active pharmaceutical ingredient (API) by biotransformation [1], the complexation into the cavity of a complexing agent [2] or layering with coating substances [3] and other methods.
Among all these solutions, coating seems to be one of the best understood and most flexible methods. In terms of the API bitterness, mild and extreme bitter APIs are applicable. Additionally, coating [4] is well suited for high dosed API formulations. There are two main approaches: (1) direct coating of the bitter API particles in multiple unit pellet system (MUPS) tablets, or (2) global coating of the tablet which contains the bitter API.
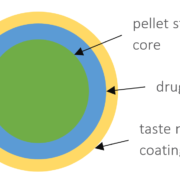
In this case study, we focus on the direct coating of API in a formulation based on starter cores as the unit pellet system. CELLETS® 200 are used as starter cores. They are pellets made of microcrystalline cellulose in size ranges from 100 µm to 1400 µm. In this specific case study, the core size ranges between 100 µm and 200 µm with a defined size distribution. The main advantages of CELLETS® are high sphericity, low friability and optimum in hardness. An API is coated onto the starter cores with a drug load of 5-20 %. The taste-masking coating is performed with Eudragit EPO® so that the resulting particle size remains below 500 µm (Figure 1). Eudragit is the polymer of choice as it ideally prevents API release in oral cavity and allows its release in the absorption window of the API. The final dosage form is a MUPS tablet (Figure 2).
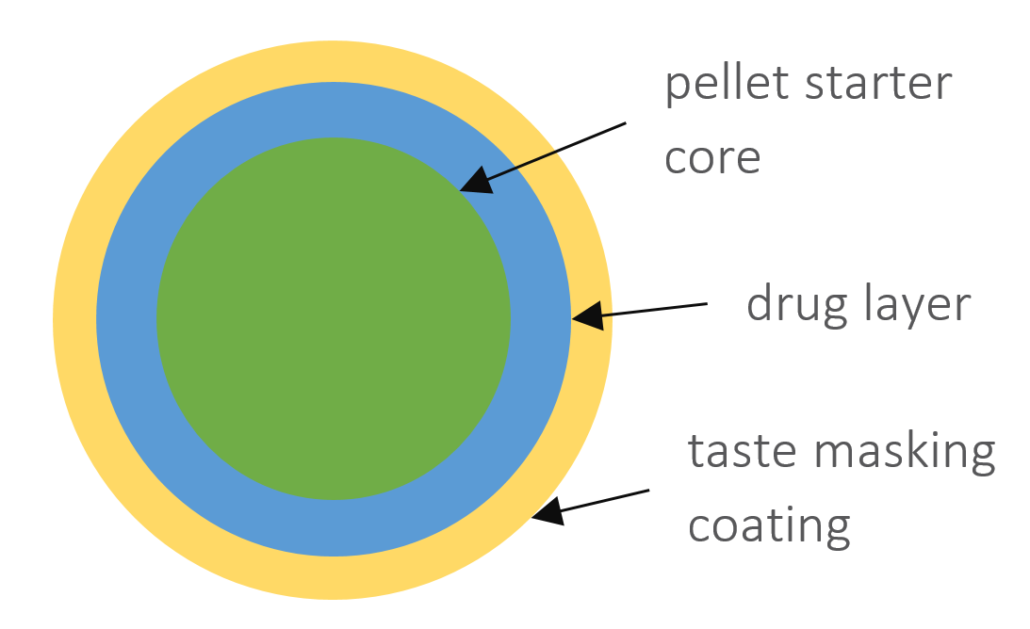
Sketch of a coated pellet starter core (green) with API (blue) and taste masking Eudragit EPO® (yellow) coating. The API drug load is between 5 % to 20 %. The resulting particle size remains below 500 µm.
Compaction is an issue during production, which requires mechanically stable pellets. Coated CELLETS® 200 after compaction still represent as “hard” core pellets. The coated pellets show only little deformation, a reversible compaction behavior and an intact coating.
Sketch of a MUPS tablet. Coated starter cores (yellow) and filling excipient formulation (white).
Here, the MUPS formulation defines a content uniformity with an excellent taste masking at pH 7 (as a measure for the oral cavity) and fast dissolution at pH 1 (representing conditions in the stomach). Figure 3 shows the evaluation of taste masking and dissolution efficiency tests, where the dissolution at pH 7 remains less than 10 % after 10 minutes, and a desired dissolution at pH 1 with a drug release better than 85 % after 30 minutes.
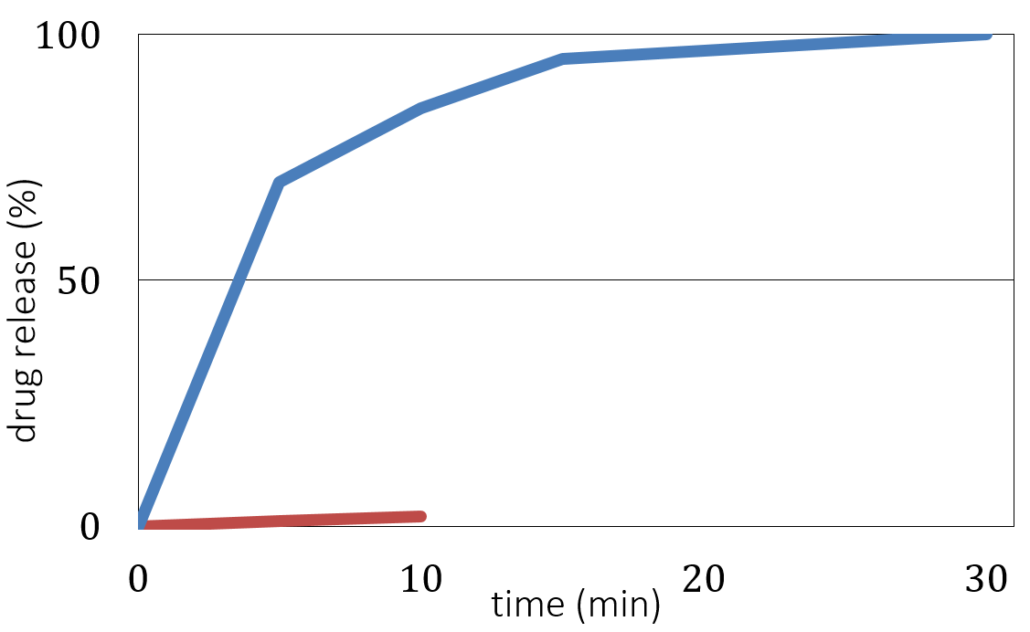
Drug release versus time for coated CELLETS® 200 starter cores. Blue line: in vitro dissolution testing at pH 1 (stomach). Red line: taste masking evaluation at pH 7 (oral cavity).
Summary
Taste masking and modification is crucial for costumer compliance in paediatric and geriatric applications. Several methods are available, such as direct API coating. CELLETS® are perfect starter cores for a successful formulation and simplify taste masking of extreme bitter APIs. CELLETS® properties allow perfect coating abilities due to a high degree of sphericity. CELLETS® of small sizes and narrow size distribution are applicable in MUPS tablets thanks to their low friability and excellent hardness.
References
[4] Hazzah, H.A., EL-Massik, M.A., Abdallah, O.Y. et al. Preparation and characterization of controlled-release doxazosin mesylate pellets using a simple drug layering-aquacoating technique. Journal of Pharmaceutical Investigation 43 (2013) 333–342. https://doi.org/10.1007/s40005-013-0077-0
Abstract
Starter beads such as pellets made of microcrystalline cellulose (MCC) are frequently used in the formulation of oral drug delivery systems, e.g. multiparticulates [1] or multi-unit pellet system (MUPS) tablets [2]. Certain properties are requested to MCC pellets. We shed some light on sphericity size and friability in this note.
Starter beads for MUPS tablets
MUPS tablets consist of pellets which are compressed – assisted by excipients such as disintegrants and fillers. The pellets used are usually functional coated to achieve desired drug release profiles.
Top: Inert Cellets® 100 (100-200 µm, left) in comparison with another MCC sphere (75-212 µm, right). Bottom: Inert Cellets® 200 (200-350 µm, left) in comparison with another MCC sphere (150-300 µm).
Figure 1: Top: Inert Cellets® 100 (100-200 µm, left) in comparison with another MCC sphere (75-212 µm, right). Bottom: Inert Cellets® 200 (200-350 µm, left) in comparison with another MCC sphere (150-300 µm).
The characteristics of the starter bead as a neutral carrier should therefore include high sphericity (Figure 1), constant particle size distribution and smooth surface. These aspects count especially for the formulation of low dosed highly active APIs.
For the application in MUPS tablets small size and high mechanical stability (low friability) are of interest to achieve desired drug loading and avoid film damage during compression.
Size
Any question relating to optimized drug load and coating layers of pellets is a question of size and sphericity of the starter beads.
So, what is the main influence of size? Size needs to be considered for achieving desired drug load in relation to a total dimension of the pellet. While the total dimension of the pellet is mainly defined by the application – e.g. processing as a capsule, tablet or sachet –, the initial pellet size defines the maximum thickness of coating levels (Figure 2). Size might also be a matter of content uniformity with low dosed API and also needs to be mentioned by means of processability, which is in particular electrostatic loading or sticking. Particle size distribution influences the dissolution profile.

Figure 2: Sketch of a functionally coated pellet. The size of the initial pellet (green) defines the maximum thickness of all coating layers (blue) which may contain API and excipients, as well.
Figure 2: Sketch of a functionally coated pellet. The size of the initial pellet (green) defines the maximum thickness of all coating layers (blue) which may contain API and excipients, as well.
Sphericity
Sphericity is a strong parameter which influence depends on drug loading and coating levels. Also for the control of dissolution profile where specific surface area and content uniformity play important roles, the influence of sphericity needs to be understood (Figure 3). Please do not forget, that with decreasing sphericity, the flow probabilities of powders are decreasing (powder rheology), which might affect process properties such as powder transport.

Figure 3: Sketch of non-spherical starter beads (green) with coating layers (blue). Coating layer thickness and dissolution profiles are hard to control in this case.
Figure 3: Sketch of non-spherical starter beads (green) with coating layers (blue). Coating layer thickness and dissolution profiles are hard to control in this case.
Thus, starter beads of uniform size (distribution) and sphericity are the better solution for overcoming these issues by simplifying drug formulation and processing. Such starter beads can be pellets of MCC, sugar or tartaric acid. MCC pellets surely show perfect initial conditions as they exhibit chemical inertness and therefore can be combined with several APIs. In case of weakly basic APIs, tartaric acid pellets are advantageous.

Figure 4: A pellet inside a compressed MUPS tablet. The starter bead is surrounded by a coating layer of exemplarily excipient or API. A powdery excipients matrix surrounds the coated pellet. Friability is absolutely low.
Figure 4: A pellet inside a compressed MUPS tablet. The starter bead is surrounded by a coating layer of exemplarily excipient or API. A powdery excipients matrix surrounds the coated pellet. Friability is absolutely low.
Figure 4 shows a cross-section of a pellet in the matrix of a compressed MUPS tablet. It is mentionable, that due to low friability a high degree of sphericity as well as surface smoothness are kept after compression and film damage of coating layers is not identified.
Summary
Cellets® offer a perfect combination of chemical inertness towards the selection of the API and physical properties that allow optimized and stable processing in a fluid bed process for layering and coating of the starter beads. Main advantages are the low friability, smooth surface, sphericity and narrow size distributions.
Cellets® starter beads therefore provide excellent conditions for controlled drug dissolution profiles.
Acknowledgement
We acknowledge Fraunhofer IFAM (Dresden, Germany) for providing electron microscopic images.
References
[1] Pöllinger N, Drug Product Development for Older Adults—Multiparticulate Formulations. In: Stegemann S. (eds) Developing Drug Products in an Aging Society. AAPS Advances in the Pharmaceutical Sciences Series, vol 26 (2016). Springer, Cham. https://doi.org/10.1007/978-3-319-43099-7_16
[2] Bhad ME, Abdul S, Jaiswal SB, Chandewar AV, Jain JM, Sakarkar DM. MUPS tablets—a brief review. Int J Pharm Tech Res. 2010;2:847–55