CELLETS® are pellets or spheres made of microcrystalline cellulose. The size ranges from 100 µm to 1400 µm. Being neutral starter cores, they can be used as carrier system for low-dosed APIs and allow diverse functional coating. See pellet technologies for a detailed description.
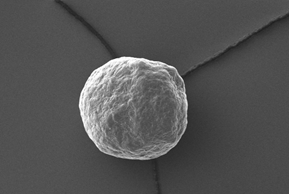
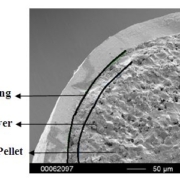
Electron microscopy yield perfect imaging data of the MCC pellets’ surfaces. Magnification: 250x, working distance 8.0 mm, voltage: 10 keV.
Available size classes are (click for more information):
- CELLETS® 100
- CELLETS® 200
- CELLETS® 350
- CELLETS® 500
- CELLETS® 700
- CELLETS® 1000
Any size class of CELLETS® have same striking advantages:
- low friability and extreme hardness
- insolubility in water
- high spherictity
- smooth surface
- good monodispersity
See case studies to see these starter pellets in action!
Abstract
Pellets are one of multiparticulate pharmaceutical forms and can offer numerous technical and biopharmaceutical advantages compared with single dose unit formulations, e.g. tablets and capsules. This study aimed at formulation of controlled-release pellets of doxazosin mesylate (DM), a widely used treatment for antihypertensive and benign prostatic hyperplasia. DM was loaded onto microcrystalline cellulose CELLETS® pellets using hydroalcoholic solution and alcoholic suspension layering techniques to achieve a minimum drug load of 4 mg DM/g pellets. DM-layered CELLETS were coated by Aquacoat dispersion (ready-made ethylcellulose dispersion) using a coating pan technique as a simple and widely utilized technique in pharmaceutical industry. Controlled-release DM-layered pellets showed a release profile comparable to the controlled-release commercial product Cardura® XL Tablet. Also, the mechanism of DM release from Aquacoat CELLETS® was mathematically modeled and imaged by scanning electron microscopy to elucidate drug release mechanisms from the prepared pellet formulations. Accelerated stability studies of the prepared pellets were performed under stress conditions of 40 °C, and 75 % RH for 3 months. In conclusion, preparation of controlled-release DM-layered CELLETS® is feasible using a simple and conventional coating pan technology. Read more about controlled-release doxazosin mesylate pellets.
References
H. A. Hazzah, M. A. EL-Massik, O. Y. Abdallah & H. Abdelkader, Journal of Pharmaceutical Investigation (2013), 43:333–342. doi:10.1007/s40005-013-0077-0
Additional information
CELLETS® are perfect starter beads for coating and layering of API, such as doxazosin mesylate. Multilayer formulation attempts enable defined release profiles and improved bioavailability. Check different pellet sizes from 100 µm to 1400 µm which fit to your formulation.
Need formulation services?
Contact our partner Glatt Pharmaceutical Services!
Abstract
Bioavailability of weakly basic drugs may be disrupted by dramatic pH changes or unexpected pH alterations in the gastrointestinal tract. Conventional organic acids or enteric coating polymers cannot address this problem adequately because they leach out or dissolve prematurely, especially during controlled release applications. Thus, a non-leachable, multifunctional terpolymer nanoparticle (TPN) made of cross-linked poly(methacrylic acid) (PMAA)-polysorbate 80-grafted-starch (PMAA-PS 80-g-St) was proposed to provide pH transition-independent release of a weakly basic drug, verapamil HCl (VER), by a rationally designed bilayer-coated controlled release bead formulation. The pH-responsive PMAA and cross-linker content in the TPN was first optimized to achieve the largest possible increase in medium uptake alongside the smallest decrease in drug release rate at pH 6.8, relative to pH 1.2. Such TPNs maintained an acidic microenvironmental pH (pHm) when loaded in ethylcellulose (EC) films, as measured using pH-indicating dyes. Further studies of formulations revealed that with the 1:2 VER:TPN ratio and 19% coating weight gain, bilayer-coated beads maintained a constant release rate over the pH transition and exhibited extended release up to 18 h. These results demonstrated that the multifunctional TPN as a pHm modifier and pH-dependent pore former could overcome the severe pH-dependent solubility of weakly basic drugs.
Introduction
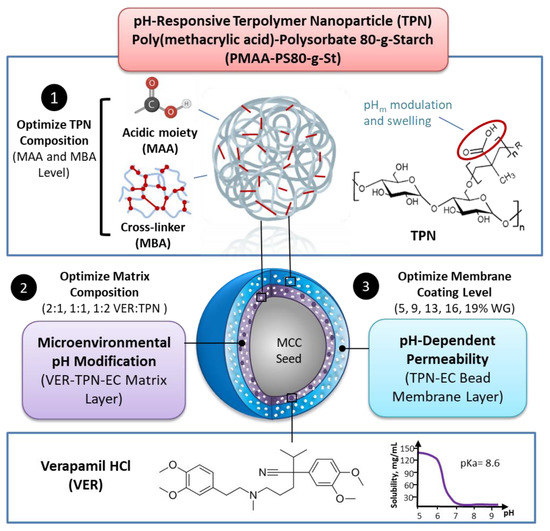
Materials and Methods
2.1. Materials
Conclusions
Disclaimer
References
- Dvořáčková, K.; Doležel, P.; Mašková, E.; Muselík, J.; Kejdušová, M.; Vetchý, D. The effect of acid pH modifiers on the release characteristics of weakly basic drug from hydrophlilic-lipophilic matrices. AAPS Pharmscitech 2013, 14, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Bassi, P.; Kaur, G. pH modulation: A mechanism to obtain pH-independent drug release. Expert Opin. Drug Deliv. 2010, 7, 845–857. [Google Scholar] [CrossRef]
- Sun, Y.; Koyama, Y.; Shimada, S. Measurement of intraluminal pH changes in the gastrointestinal tract of mice with gastrointestinal diseases. Biochem. Biophys. Res. Commun. 2022, 620, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Vinarov, Z.; Abdallah, M.; Agundez, J.A.G.; Allegaert, K.; Basit, A.W.; Braeckmans, M.; Ceulemans, J.; Corsetti, M.; Griffin, B.T.; Grimm, M.; et al. Impact of gastrointestinal tract variability on oral drug absorption and pharmacokinetics: An UNGAP review. Eur. J. Pharm. Sci. 2021, 162, 105812. [Google Scholar] [CrossRef] [PubMed]
- Thoma, K.; Ziegler, I. The pH-independent release of fenoldopam from pellets with insoluble film coats. Eur. J. Pharm. Biopharm. 1998, 46, 105–113. [Google Scholar] [CrossRef]
- Bolourchian, N.; Dadashzadeh, S. PH-independent release of propranolol hydrochloride from HPMC-based matrices using organic acids. Daru 2008, 16, 136–142. [Google Scholar]
- Nie, S.; Pan, W.; Li, X.; Wu, X. The effect of citric acid added to hydroxypropyl methylcellulose (HPMC) matrix tablets on the release profile of vinpocetine. Drug Dev. Ind. Pharm. 2004, 30, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, R.; Hong, E.; Villafuerte, L. Influence of admixed citric acid on the release profile of pelanserin hydrochloride from HPMC matrix tablets. Int. J. Pharm. 2000, 201, 165–173. [Google Scholar] [CrossRef]
- Streubel, A.; Siepmann, J.; Dashevsky, A.; Bodmeier, R. pH-independent release of a weakly basic drug from water-insoluble and -soluble matrix tablets. J. Control. Release 2000, 67, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Obaidat, A. Modulation of the micro-environmental pH and its influence on the gel layer behavior and release of theophylline from hydrophilic matrices. Int. J. Pharma. Bio Sci. 2013, 4, P794–P802. [Google Scholar]
- Siepe, S.; Lueckel, B.; Kramer, A.; Ries, A.; Gurny, R. Assessment of tailor-made HPMC-based matrix minitablets comprising a weakly basic drug compound. Drug Dev. Ind. Pharm. 2008, 34, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Siepe, S.; Lueckel, B.; Kramer, A.; Ries, A.; Gurny, R. Strategies for the design of hydrophilic matrix tablets with controlled microenvironmental pH. Int. J. Pharm. 2006, 316, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Yoshioka, M.; Horibe, H.; Hirai, S.; Kitamori, N.; Toguchi, H. pH-independent controlled-release microspheres using polyglycerol esters of fatty acids. J. Pharm. Sci. 1994, 83, 1600–1607. [Google Scholar] [CrossRef]
- Lecomte, F.; Siepmann, J.; Walther, M.; MacRae, R.J.; Bodmeier, R. Blends of enteric and GIT-insoluble polymers used for film coating: Physicochemical characterization and drug release patterns. J. Control. Release 2003, 89, 457–471. [Google Scholar] [CrossRef]
- Sakellariou, P.; Rowe, R.C.; White, E.F.T. Polymer/polymer interaction in blends of ethyl cellulose with both cellulose derivatives and polyethylene glycol 6000. Int. J. Pharm. 1986, 34, 93–103. [Google Scholar] [CrossRef]
- Sakellariou, P.; Rowe, R.C. Phase separation and morphology in ethylcellulose/cellulose acetate phthalate blends. J. Appl. Polym. Sci. 1991, 43, 845–855. [Google Scholar] [CrossRef]
- Lecomte, F.; Siepmann, J.; Walther, M.; MacRae, R.J.; Bodmeier, R. pH-Sensitive polymer blends used as coating materials to control drug release from spherical beads: Elucidation of the underlying mass transport mechanisms. Pharm. Res. 2005, 22, 1129–1141. [Google Scholar] [CrossRef]
- Khan, M.Z.; Prebeg, Z.; Kurjaković, N. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers. I. Manipulation Of drug release using Eudragit L100-55 and Eudragit S100 combinations. J. Control. Release 1999, 58, 215–222. [Google Scholar] [CrossRef]
- John, R.; Howard, P.T. Controlled Release Formulation. U.S. Patent US4792452A, 20 December 1988. [Google Scholar]
- Amighi, K.; Timmermans, J.; Puigdevall, J.; Baltes, E.; Moës, A.J. Peroral sustained-release film-coated pellets as a means to overcome physicochemical and biological drug-related problems. I. In vitro development and evaluation. Drug Dev. Ind. Pharm. 1998, 24, 509–515. [Google Scholar] [CrossRef]
- Gruber, P.; Brickl, R.; Bozler, G.; Stricker, H. Dipyricamole Sustained Release Forms Comprising Lacquer-Coated Particles and the Preparation Thereof. U.S. Patent US4367217A, 4 January 1983. [Google Scholar]
- Cole, E.T.; Scott, R.A.; Connor, A.L.; Wilding, I.R.; Petereit, H.U.; Schminke, C.; Beckert, T.; Cadé, D. Enteric coated HPMC capsules designed to achieve intestinal targeting. Int. J. Pharm. 2002, 231, 83–95. [Google Scholar] [CrossRef]
- Felton, L.A.; Porter, S.C. An update on pharmaceutical film coating for drug delivery. Expert Opin. Drug. Deliv. 2013, 10, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Frohoff-Hülsmann, M.A.; Lippold, B.C.; McGinity, J.W. Aqueous ethyl cellulose dispersion containing plasticizers of different water solubility and hydroxypropyl methyl-cellulose as coating material for diffusion pellets II: Properties of sprayed films. Eur. J. Pharm. Biopharm. 1999, 48, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Luliński, P. Molecularly imprinted polymers based drug delivery devices: A way to application in modern pharmacotherapy. A review. Mater. Sci. Eng. C 2017, 76, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, M.; Mohammadi, A.; Manshadi, M.K.D.; Sanati-Nezhad, A. Mathematical and computational modeling of nano-engineered drug delivery systems. J. Control. Release 2019, 307, 150–165. [Google Scholar] [CrossRef]
- Al Ragib, A.; Chakma, R.; Dewan, K.; Islam, T.; Kormoker, T.; Idris, A.M. Current advanced drug delivery systems: Challenges and potentialities. J. Drug Deliv. Sci. Technol. 2022, 76, 103727. [Google Scholar] [CrossRef]
- Davoodi, P.; Lee, L.Y.; Xu, Q.; Sunil, V.; Sun, Y.; Soh, S.; Wang, C.-H. Drug delivery systems for programmed and on-demand release. Adv. Drug Deliv. Rev. 2018, 132, 104–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cheung, R.; Wu, X.Y.; Ballinger, J.R.; Bendayan, R.; Rauth, A.M. A study of doxorubicin loading onto and release from sulfopropyl dextran ion-exchange microspheres. J. Control. Release 2001, 77, 213–224. [Google Scholar] [CrossRef]
- Wong, H.L.; Bendayan, R.; Rauth, A.M.; Wu, X.Y. Development of solid lipid nanoparticles containing ionically complexed chemotherapeutic drugs and chemosensitizers. J. Pharm. Sci. 2004, 93, 1993–2008. [Google Scholar] [CrossRef]
- Shalviri, A.; Raval, G.; Prasad, P.; Chan, C.; Liu, Q.; Heerklotz, H.; Rauth, A.M.; Wu, X.Y. pH-Dependent doxorubicin release from terpolymer of starch, polymethacrylic acid and polysorbate 80 nanoparticles for overcoming multi-drug resistance in human breast cancer cells. Eur. J. Pharm. Biopharm. 2012, 82, 587–597. [Google Scholar] [CrossRef]
- Shalviri, A.; Chan, H.K.; Raval, G.; Abdekhodaie, M.J.; Liu, Q.; Heerklotz, H.; Wu, X.Y. Design of pH-responsive nanoparticles of terpolymer of poly(methacrylic acid), polysorbate 80 and starch for delivery of doxorubicin. Colloids Surf. B Biointerfaces 2013, 101, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chang, H.H.R.; Shalviri, A.; Li, J.; Lugtu-Pe, J.A.; Kane, A.; Wu, X.Y. Investigation of a new pH-responsive nanoparticulate pore former for controlled release enteric coating with improved processability and stability. Eur. J. Pharm. Biopharm. 2017, 120, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.I.; Gomes, E.C.L.; Soares, C.D.V.; Cunha, A.F.; Oliveira, M.A. Thermal Analysis Applied to Verapamil Hydrochloride Characterization in Pharmaceutical Formulations. Molecules 2010, 15, 2439–2452. [Google Scholar] [CrossRef] [PubMed]
This article “Amorphous Solid Dispersions Layered onto Pellets – An Alternative to Spray Drying?” is an excerpt from the publication of Neuwirth et al., Pharmaceutics 2023, 15(3), 764; https://doi.org/10.3390/pharmaceutics15030764.
Abstract
Materials
| Pellet Properties | |
|---|---|
| d50 (xc min) [µm] | 1123.44 (±7.36) |
| SPAN | 0.166 (±0.002) |
| b/l | 0.893 (±0.000) |
| SPHT | 0.956 (±0.001) |
| Particle density [g/cm3] | 1.452 (±0.016) |
| Sm [cm2/g] | 36.41 (±0.29) |
Table 1. Pellet properties of Cellets 1000. d50: mean particle diameter determined by the particle width; SPAN: width of the particle distribution; b/l: aspect ratio; SPHT: sphericity; Sm: specific surface area.
Conclusions
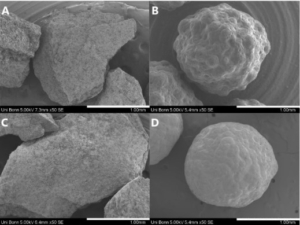
Figure: Amorphous solid dispersions.
[1] Neuwirth et al., Pharmaceutics 2023, 15(3), 764; https://doi.org/10.3390/pharmaceutics15030764
Introduction on amorphous solid dispersions
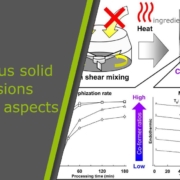
What is the benefit of multilayer amorphous solid dispersions? Recently, several studies had been performed on amorphous solid dispersions working spheres or starter beads. Starter beads, such as MCC (Microcrystalline Cellulose) spheres are employed due to their high friability and chemical inertness. Some studies are even working on solventless pelletization and amorphization using high shear granulator techniques [1].
Amorphization of poorly water-soluble drugs is a promising approach to improve the solubility and dissolution rate as amorphous solids lack a crystal lattice with long-range order [2]. Unfortunately, a high chemical potential compared to crystalline forms makes amorphous forms thermodynamically unstable. Thus, amorphous drugs exhibit low physical stability and finally lack of recrystallization [3,4]. In turn, surface crystallization is to be minimized.
Multilayer amorphous solid dispersions
This is the key focus of a publication by Eline Boel and Guy Van den Mooter: They had been investigating a promising solution of multilayer high-drug load amorphous solid dispersions, as follows [5]:
Inhibiting surface crystallization is an interesting strategy to enhance the physical stability of amorphous solid dispersions (ASDs), still preserving high drug loads. The aim of this study was to investigate the potential surface crystallization inhibitory effect of an additional polymer coating onto ASDs, comprising high drug loads of a fast crystallizing drug, layered onto pellets. For this purpose, bilayer coated pellets were generated with fluid-bed coating, of which the first layer constitutes a solid dispersion of naproxen (NAP) in poly(vinylpyrrolidone-co-vinyl acetate) (PVP-VA) in a 40:60 or 35:65 (w/w) ratio, and ethyl cellulose (EC) composes the second layer. The physical stability of these double-layered pellets, in comparison to pellets with an ASD layer only, was assessed under accelerated conditions by monitoring with X-ray powder diffraction (XRPD) at regular time intervals. Bilayer coated pellets were however found to be physically less stable than pellets with an ASD layer only. Applying the supplementary EC coating layer induced crystallization and heterogeneity in the 40:60 and 35:65 (w/w) NAP-PVP-VA ASDs, respectively, attributed to the initial contact with the solvent. Caution is thus required when applying an additional coating layer on top of an ASD layer with fluid-bed coating, for instance for controlled release purposes, especially if the ASD consists of high loads of a fast crystallizing drug.
Read more on doi:10.1016/j.ijpharm.2022.122455.
How about following up studies on ASD formulation with starter beads? Simply, contact us für MCC spheres, such as CELLETS® 700 (700-1000 µm, US mesh 18/25).
Your technology and formulation partner for amorphous solid dispersions:
References
[1] K. Kondo, T. Rades, European Journal of Pharmaceutics and Biopharmaceutics 181 (2022) 183–194 doi:10.1016/j.ejpb.2022.11.011
[2] B.C. Hancock, M. Parks, Pharm. Res. 17 (2000) 397-404.
[3] L.I. Blaabjerg, E. Lindenberg, T. Rades, H. Grohganz, K. Lobmann, Int. J. Pharm. 521 (2017) 232-238.
[4] A. Singh, G. Van den Mooter, Adv. Drug Deliv. Rev. 100 (2016) 27-50.
[5] E. Boel, G. Van den Mooter, International Journal of Pharmaceutics (2022) 122455. doi:10.1016/j.ijpharm.2022.122455
We identified, that amorphous solid dispersions gain in importance as they increase the solubility and dissolution rate of poorly water-soluble drugs. There are severall attempts, in which each of them positive aspects and certain issues occur. It’s time, drawing amorphous solids dispersions in a more general context and sheding some more light on elementary aspects. We like to point on an excellent summary given by Thomas Rades and Keita Kondo [Rades_2022]. Before switching over, we like to emphasis and anticipate one message: The lastest attempts for amorphous solid dispersions is using CELLETS® 175 (MCC spheres) which do not only act as drug carrier, but – due to best friability – as milling balls. You might like follow this attempt with MCC starter beads, so please contact us for getting some materials. Let’s now read more from Rades et al.:
Draw-back on Amorphous solid dispersions
Amorphization is a promising approach to improve the solubility and dissolution rate of poorly water-soluble drugs as amorphous solids lack a crystal lattice with long-range order [1]. However, since amorphous forms are thermodynamically unstable due to a high chemical potential compared to crystalline forms, amorphous drugs exhibit low physical stability and finally recrystallizes [2], [3]. Thus, strategies to stabilize amorphous forms are essential in the development of amorphous products and include the design of amorphous solid dispersions (ASDs) [4], [5] and co-amorphous formulations [6], [7], [8]. ASDs are the most common approach for preparing amorphous products and involve glass formation by molecularly dispersing drug compounds into an amorphous polymer [4], [5]. However, ASD preparations may require a large quantity of polymer to stabilize amorphized drug due to their low miscibility with drug molecules [9], leading to a high bulk volume of the amorphous products. Co-amorphous systems have recently attracted attention as an alternative approach to amorphous formulations and include the formation of a single amorphous phase in which multiple low molecular weight initially crystalline materials (including drug compounds) are uniformly mixed at the molecular levels [6], [7], [8]. Co-amorphous mixtures typically exhibit high physical stability and dissolution characteristics [6], [7], [10].
Co-amorphous systems are typically classified as drug-drug combinations and drug-excipient mixtures. In the former combinations, amorphous phases comprise two drug compounds, which act as a stabilizer for each other by forming intermolecular interactions [11], [12], [13]. These formulations are expected to offer a combination of drugs to enhance the therapeutic effects but their applicability is limited as drug-drug combinations forming co-amorphous solids are not necessarily suitable for combination therapy, or require fixed doses, not necessarily suitable for co-amorphization. In the co-amorphous drug-excipient systems, low molecular-weight substances (including organic acids [14], sugars [15] and amino acids [16]) act as a co-former and their properties and mixing ratio with the drug affect dissolution characteristics and physical stability of the resulting co-amorphous mixtures [8], [10]. Recently, various combinations of drug compounds and amino acids were systematically investigated [17], [18], indicating that co-amorphous mixtures with high dissolution characteristics and physical stability can be produced by selecting amino acids that can form interactions with the target drug compounds (e.g. pairs of acidic drugs and basic amino acids). Therefore, amino acids are a promising co-former class for co-amorphous formulations.
Preparation of co-amorphous mixtures has been reported using melt quenching [13], [19], spray drying [20], [21], and ball milling [16], [22]. Since the resulting solids are in cake or powder forms (regardless of the preparation method), downstream processes including milling and granulation are usually essential to produce final dosage forms such as capsules and tablets for oral administration [23]. These processes can lead to increased risk of phase separations and crystallization due to moisture, thermal, and mechanical stresses. In ASD systems, to avoid the problems due to the downstream processes, one-step preparations of ASD granules by amorphizing drug compounds during the granulation process using fluidized bed processors [24], [25], [26], [27], [28], [29], [30] and high shear granulators [31], [32], [33], [34] have been investigated. However, to our knowledge, there are no reports on one-step preparation methods for co-amorphous granules. In the first part of the current study, we investigated the feasibility of solventless amorphization and pelletization using a high shear granulator and could obtain fully amorphized indomethacin-layered pellets by simply mixing indomethacin crystals and microcrystalline cellulose spheres without using solvent and heating. Indomethacin crystals were pulverized and amorphized due to collisions with the spheres and subsequently are deposited on the surface of the spheres. Therefore, we hypothesized that co-amorphous mixture-layered pellets can be produced through a one-step amorphization and pelletization process using this technique, as the preparation of co-amorphous mixtures has previously been performed by mechanical activation [16], [22]. Furthermore, this technique holds promise as an economical as well as sustainable approach to manufacture co-amorphous formulations as the need for solvent and/or heat is eliminated.
In previous research, various combinations of indomethacin and amino acids for co-amorphous preparations were systematically investigated. The findings suggest that arginine is an excellent co-former for indomethacin to prepare co-amorphous mixtures with fast dissolution characteristics and high physical stability [18], as an amorphous salt is formed due to strong interactions between the acidic drug indomethacin and the basic amino acid arginine [35], [36]. In the current study, to investigate whether co-amorphous layer pellets can be produced through a one-step amorphization and pelletization process, indomethacin and arginine were selected as the model drug and the co-former, respectively. In the first part of this study, indomethacin crystals were mixed with microcrystalline cellulose spheres (with various mean diameters of 140 μm, 195 μm, 275 μm, 414 μm, and 649 μm) at a weight ratio of 1:10 using a high shear granulator [added: TMG1/6, Glatt GmbH, Binzen, Germany]. Fully amorphized indomethacin-layered pellets were obtained using carriers of 414 μm in diameter, whereas partially amorphized indomethacin-layered pellets were obtained using carriers of 195 μm in diameter. This difference was likely due to the higher impact forces of larger carriers promoting mechanical activation of indomethacin crystals. In this part of the study, to clarify the effects of using arginine on the amorphization and pelletization of indomethacin, the smaller cellulose spheres of 195 μm in diameter were employed as carrier particles. Indomethacin crystals and arginine crystals were initially mixed at various molar ratios (1:1, 2:1, and 3:1), and then the resulting mixtures were high shear granulated with microcrystalline cellulose spheres at a weight ratio of 1:10. The resulting composite particles were characterized using solid-state and particle analytical techniques. To identify effective co-amorphization approaches, we examined high shear mixing under various jacket temperatures. Furthermore, physical stability and dissolution characteristics of co-amorphous layer pellets were investigated.
References
[Rades_2022] K. Kondo, T. Rades, 181 (2022) 183-194. doi:10.1016/j.ejpb.2022.11.011
[1] B.C. Hancock, M. Parks, Pharm. Res. 17 (2000) 397-404.
[2] L. Yu, Adv. Drug Deliv. Rev. 48 (2001) 27-42.
[3] L.R. Hilden, K.R. Morris, J. Pharm. Sci. 93 (2004) 3-12.
[4] T. Vasconcelos, S. Marques, J. das Neves, B. Sarmento, Adv. Drug Deliv. Rev. 100 (2016) 85-101.
[5] S. Baghel, H. Cathcart, N.J. O’Reilly, J. Pharm. Sci. 105 (2016) 2527-2544.
[6] R. Laitinen, K. Lobmann, C.J. Strachan, H. Grohganz, T. Rades, Int. J. Pharm. 453 (2013) 65-79.
[7] R.B. Chavan, R. Thipparaboina, D. Kumar, N.R. Shastri, Int. J. Pharm. 515 (2016) 403-415.
[8] S.J. Dengale, H. Grohganz, T. Rades, K. Lobmann, Adv. Drug Deliv. Rev. 100 (2016) 116-125.
[9] S. Janssens, G. Van den Mooter, J. Pharm. Pharmacol. 61 (2009) 1571-1586.
[10] R. Laitinen, K. Lobmann, H. Grohganz, P. Priemel, C.J. Strachan, T. Rades, Int. J. Pharm. 532 (2017) 1-12.
[11] S. Yamamura, H. Gotoh, Y. Sakamoto, Y. Momose, Eur. J. Pharm. Biopharm. 49 (2000) 259-265.
[12] M. Allesø, N. Chieng, S. Rehder, J. Rantanen, T. Rades, J. Aaltonen, J. Control. Release 136 (2009) 45-53.
[13] K. Lobmann, R. Laitinen, H. Grohganz, K.C. Gordon, C. Strachan, T. Rades, Mol. Pharm. 8 (2011) 1919-1928.
[14] Q. Lu, G. Zografi, Pharm. Res. 15 (1998) 1202-1206.
[15] M. Descamps, J.F. Willart, E. Dudognon, V. Caron, J. Pharm. Sci. 96 (2007) 1398-1407.
[16] K. Lobmann, H. Grohganz, R. Laitinen, C. Strachan, T. Rades, Eur. J. Pharm. Biopharm. 85 (2013) 873-881.
[17] G. Kasten, H. Grohganz, T. Rades, K. Lobmann, Eur. J. Pharm. Sci. 95 (2016) 28-35.
[18] G. Kasten, K. Lobmann, H. Grohganz, T. Rades, Int. J. Pharm. 557 (2019) 366-373.
[19] A. Teja, P.B. Musmade, A.B. Khade, S.J. Dengale, Eur. J. Pharm. Sci. 78 (2015) 234-244.
[20] A. Beyer, L. Radi, H. Grohganz, K. Lobmann, T. Rades, C.S. Leopold, Eur. J. Pharm. Biopharm. 104 (2016) 72-81.
[21] E. Lenz, K.T. Jensen, L.I. Blaabjerg, K. Knop, H. Grohganz, K. Lobmann, T. Rades,
- Kleinebudde, Eur. J. Pharm. Biopharm. 96 (2015) 44-52.
[22] K.T. Jensen, F.H. Larsen, C. Cornett, K. Lobmann, H. Grohganz, T. Rades, Mol. Pharm. 12 (2015) 2484-2492.
[23] B. Demuth, Z.K. Nagy, A. Balogh, T. Vigh, G. Marosi, G. Verreck, I. Van Assche, M.E. Brewster, Int. J. Pharm. 486 (2015) 268-286.
[24] D.B. Beten, K. Amighi, A.J. Möes, Pharm. Res. 12 (1995) 1269-1272.
[25] H.-O. Ho, H.-L. Su, T. Tsai, M.-T. Sheu, Int. J. Pharm. 139 (1996) 223-229.
[26] N. Sun, X. Wei, B. Wu, J. Chen, Y. Lu, W. Wu, Powder Technol. 182 (2008) 72-80.
[27] A. Dereymaker, D.J. Scurr, E.D. Steer, C.J. Roberts, G. Van den Mooter, Mol. Pharm. 14 (2017) 959-973.
[28] A. Dereymaker, J. Pelgrims, F. Engelen, P. Adriaensens, G. Van den Mooter, Mol. Pharm. 14 (2017) 974-983.
[29] T. Oshima, R. Sonoda, M. Ohkuma, H. Sunada, Chem. Pharm. Bull. 55 (2007) 1557-1562.
[30] H.J. Kwon, E.J. Heo, Y.H. Kim, S. Kim, Y.H. Hwang, J.M. Byun, S.H. Cheon, S.Y. Park, D.Y. Kim, K.H. Cho, H.J. Maeng, D.J. Jang, Pharmaceutics 11(3) (2019) 136.
[31] N.S. Trasi, S. Bhujbal, Q.T. Zhou, L.S. Taylor, Int. J. Pharm. X 1 (2019) 100035.
[32] A. Seo, P. Holm, H.G. Kristensen, T. Schæfer, Int. J. Pharm. 259 (2003) 161-171.
[33] T. Vilhelmsen, H. Eliasen, T. Schaefer, Int. J. Pharm. 303 (2005) 132-142.
[34] Y.C. Chen, H.O. Ho, J.D. Chiou, M.T. Sheu, Int. J. Pharm. 473 (2014) 458-468.
[35] K.T. Jensen, L.I. Blaabjerg, E. Lenz, A. Bohr, H. Grohganz, P. Kleinebudde, T. Rades, K. Lobmann, J. Pharm. Pharmacol. 68 (2016) 615-624.
[36] K.T. Jensen, F.H. Larsen, K. Lobmann, T. Rades, H. Grohganz, Eur. J. Pharm. Biopharm. 107 (2016) 32-39.
More information on ASD
Read more about amorphous solid dispersions in our application notes.
Abstract
This case study is a short abstract on spouted bed characteristics, following closely findings in the publication by J. Vanamu and A. Sahoo [1].
Spouted bed systems are of highest importance for all powder processing industries, and more specific in pharmaceutical industry for coating and drying in pellet technologies [2]. These systems offer manufacturing particularly fine and temperature-sensitive particles from small to large scale: laboratory systems are capable of processing product volumes of very few grams, while production systems can handle capacities of several tons [3].
But how to control conditions in spouted beds for efficient process applications, like mixing, coating, or drying?
There might be certain reasons, that the hydrodynamic behavior of the spouted bed in the pharmaceutical industries is less investigated. The referred publication shed some light on the hydrodynamic characteristics of a spouted bed where the MCC Spheres (CELLETS®) are adopted as the bed material. These starter cores are ideal model systems due to their perfect sphericity and zero-level friability. At the same time, smooth and defined surface structure initiate perfect modelling conditions in the spouted bed dynamics.
Material
CELLETS®, made of 100% Microcrystalline Cellulose, have been used as bed material. The physical properties of the CELLETS® are shown in Table 1. The CELLETS® particle morphology is represented in Figure 1.
| Parameter | Value |
| CELLETS® 700 and CELLETS® 1000 | |
| Size distribution | 700-1000 µm (CELLETS® 700)
1000-1400 µm (CELLETS® 1000) |
| Bulk density | 800 kg/m3 |
| Particle sphericity | > 0.9 |
| Void fraction | 0.42 |
| Geldart classification | B |
Table 1: Physical properties of the CELLETS®.

Figure 1: SEM micrographs of CELLETS® 700, found in [1].
Spouted bed: experiment setup
There are some international players on the market of spouted bed technologies, such as Glatt which seems to be the major one (Figure 2). In this framework, a self-made setup is used for experiments. The experiments that have been carried out in a column, which is fabricated from a Perspex sheet. This column consists of a cylindrical section of height 0.53 m and a diameter of the cylinder of 0.135 m. The column further converged the diameter of the cylinder to 0.05 m as a conical bottom having a length of 0.47 m. The spouting air is supplied by a compressed air line is controlled by a gas regulator. The airflow is controlled by a gate valve and a mesh plate having a mesh size less than the size of the bed material is employed as a separator preventing the backflow of the bed material. Images are captured using a high-speed video camera to gain more details of the hydrodynamic characteristics of the flow pattern inside the spouted bed geometry.
Figure 2: Scheme of a spouted bed (Glatt, Germany).
Experiments & spouted bed results
Experiments are carried out with three different static bed heights of shallow depth wherein the bed height is in the range of factor 2-3 of the Inlet diameter using two different particle distribution classes at 500-710 µm and 700-1000 µm, respectively. Analyzed parameters are the pressure drop across the bed, the bed expansion ratio, and the clusters concerning the superficial gas velocity are focused in the following.
J. Vanamu et al. found that the “bed expansion ratio increases with increasing superficial gas velocity until the onset of external spouting, further increase in the superficial gas velocity, the bed expansion ratio decreases. With increasing the volume of bed, the bed expansion ratio decreases. In a larger volume of bed, the particles tend to spout into the freeboard region rather than expanding with higher superficial gas velocity”. Initial spouting is symmetric, but with increasing superficial gas velocity spouting becomes asymmetric, and asymmetry is more pronounced or starts at lower superficial gas velocities for smaller particles. This agrees with existing theories of hydrodynamic behavior in a fluidized environment. Respecting the necessarity of a proper flow behavior for mixing, coating or drying applications in drug processing, symmetric spouting is essential. In turn, the superficial gas velocity may be kept low.
In case that high superficial gas velocity regimes are required for the operations a draft tube may be installed within the column to achieve the symmetric spout formation.
Summary
This case study highlights the Hydrodynamic behavior of MCC spheres in a spouted bed using image processing method. MCC spheres in the range between 500-710 µm and 700-1000 µm had been employed. All spheres showed a symmetric and asymmetric spouting in the spouted bed. With increasing superficial gas velocity, the fully suspended particles are limited to a certain height in the freeboard region due to the gas-solid crossflow. A change from symmetric to asymmetric spouting is observed with increasing superficial gas velocity.
Keeping the conditions efficient for the mixing, coating or drying applications requires finally to suppress high superficial gas velocities, or changing the setup in such way, that symmetric spouting conditions are kept upright even at higher superficial gas velocities.
References
[1] J. Vanamu and A. Sahoo, Particuology 76 (2023) 101
[2] L. A. P. de Freitas, Particuology 42 (2019) 126
[3] Glatt GmbH, Binzen, Germany. Online on Nov 8, 2022: Spouted bed systems – Glatt – Integrated Process Solutions
Great thanks to Arihant Innochem Pvt. Ltd. who supplied and donated CELLETS® for this study.
Abstract
Multiparticulates made of pellets are ideal dosage forms to be used in pediatrics. Having the suitability of paediatric consumers in mind, formulations of small-sized pellets offer a valuable base for increased compliance and improved age-appropriately dosage form. Due to their round shape of pellets, smooth surface area and narrow particle size distribution they can easily be functionally coated [1] to achieve e. g. a taste masking, enteric protection or the controlled release of the active pharmaceutical ingredient (API) in defined parts of the gastro-intestinal (GI) tract. The release profile then often depends on the coating weight gain (thickness) and composition of the functional coating.
Coating weight gain, manufacture and analysis of pellets
A well soluble drug was used as model API. In a first approach, pellets were produced applying the ProCell technology, a direct pelletization process allowing the production of highly drug loaded matrix pellets (here 95%) in a spouted bed. Two types of pellets were produced: A) with a poly amino saccharide-based binder, followed by a cellulose based seal coating and B) with a polyacrylic acid-based binder, followed by a pH-depending coating. In a second approach the API was layered onto inert starter cores (MCC, CELLETS® 200) by the aid of a cellulose based binder and antitacking agent applying the Wurster technology targeting a drug load of 50 %, followed by a pH-depending coating (C). All three pellets-based populations were functionally coated by a pH-independent sustained release polymer. Samples were taken at pre-defined coating levels for dissolution testing. For API layering and coating a GPCG 1.1 with a 6” Wurster insert was used. Direct pelletization was performed in a ProCell 5. Particle size distribution (PSD) analysis was performed by Eyecon2TM. The particle size is given as numeric or volumetric distribution (e.g. Dn50 or Dv50). The specific surface area is calculated by measuring the true density by gas pycnometry and the Sauter diameter by Laser diffraction. Dissolution was measured in the acid stage (0.1 M HCl), in buffer pH 5.5 and in buffer pH 7.2 over 300 min. The API should not be released in the first 180 min. Between 210 min and 240 min an increased drug release is expected. The dissolution rates at 225 min were compared for the coating levels at 10, 15 and 20 %.
Results
With increasing coating weight gains decreasing dissolution rates at 225 min were measured for the sustained release coating with a good linearity. Matrix PEL (A) show higher dissolution rates comparing the same coating levels than Matrix PEL (B), Wurster pellets showed the strongest decrease with increasing CWG, table 1, figure 1. This correlation was not observed for pH-depending coating (data not shown).
| Dv 50 [µm] | Dn 50 [µm] | PSD mean [µm] | Specific surface area [m2/g] | ||
| A | Matrix PEL | 496 | 475 | 481 | 0,00980 |
| B | Matrix PEL | 461 | 427 | 425 | 0,01210 |
| C | Wurster PEL | 414 | 396 | 401 | 0,01100 |
Table 1. PSD data and specific surface area of starter beads before functional coating.
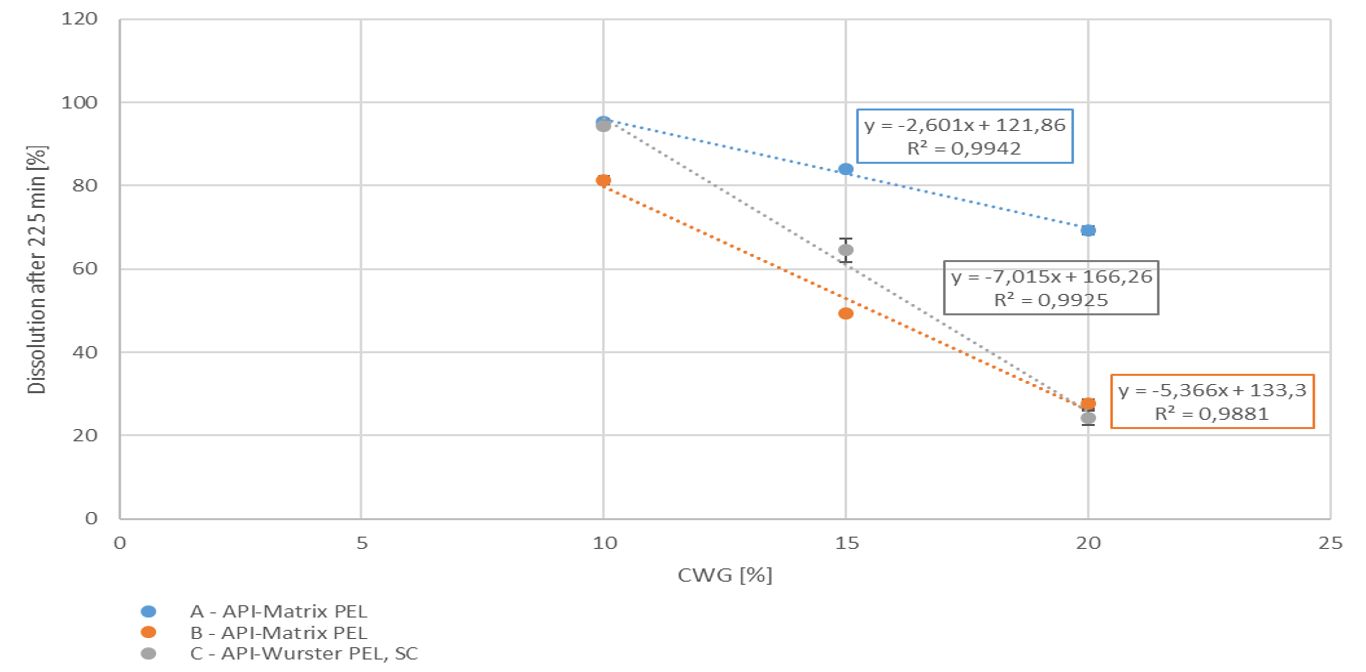
Figure 1. Dissolution at 225 min vs. coating weight gain (CWG)
Summary
Drug loaded pellets were prepared either as matrix pellets applying the ProCell technology, or by layering of starter cores applying the Wurster technology. Both populations were coated with different coating levels of a sustained release functional coating, resulting in decreasing dissolution rates with increasing coating weight gain. Due to the good correlation between coating weight gain and dissolution profile a prediction of the dissolution rate might be possible for pre-defined coating levels. These findings are a crucial step towards novel paediatric formulations with improved dissolution profiles and dosage safety.
References
[1] Palugan, L.; Cerea, M.; Zema, L.; Gazzaniga, A.; Maroni, A. Coated pellets for oral colon delivery, Journal of Drug Delivery Science and Technology 25, 1 – 15 (2015).
This study was presented on 14th annual EuPFI conference, Rome, Italy.
Abstract on Metoprolol pellets
Metoprolol Tartrate is a salt of Metoprolol, a selective β1-receptor blocker medication. The application is the treatment of high blood pressure, chest pain due to poor blood flow to the heart, and several conditions involving an abnormally fast heart rate [2]. In the following, an attempt is shown, wherein MCC spheres are used as starter cores for a multi-layer pellet formulation.
This case study is a short abstract of the publication by P. Wanasawas et al. [1] and presents controlled release Metoprolol Tartrate layered pellets achieving colon-specific drug delivery.
Pellet technology attempt
In the following, in-situ calcium pectinate-coated MCC pellets (CELLETS® 700) were proposed by applying an alternate coating method to drug-layered pellets to achieve colon-specific drug delivery. Using a centrifugal granulator, inert MCC pellets were layered by a Metoprolol Tartrate water-soluble model drug. A protective HPMC layer helps to strengthen cracks or delamination from the core in the later stage of the coating processes. Then, alternate coatings of pectin and calcium chloride layers were spray coated by fluidized bed bottom spray technology (GPCG-1, Glatt®, Germany). This technology allows achieving uniform coating layers. The subcoating with pectin and calcium pectinate polymers allow site-specific drug delivery targeting the colon due to their low water solubility. Both excipients additionally degraded completely by gut microflora [3].
By testing different composition in multilayer coatings with calcium and pectin, some interesting phenomena are stated:
- the release behavior follows the Higuchi model
- the drug release can be described by a diffusion control mechanism
- the coating of the outermost layer defines the success in controlled drug release
The latter point issues the importance of the outermost layer which is whether composed by pectin or calcium. In case of calcium, the drug release was accelerated independently of the number of Ca/P layers, such that a 4-layer system (P/Ca/P/Ca) yield a faster drug release that a 3-alyer system (P/Ca/P), see Figure 1. This is explained by the effect of the calcium ions in the outermost layer, leading to a weakening in the calcium pectinate coating layer.

Figure 1: Metoprolol pellets. From left to right: Ca/P, P/Ca/P, Ca/P/Ca/P layered pellets. Colors: Cellets as MCC pellet (green), Metoprolol Tartrate (orange), Talcum (blue), Calcium (white), Pectin (grey).
Once, pectin is the component in the outermost layer, this led to a difference in drug release at neutral and slightly acidic conditions of the dissolution media. While in a neutral pH 7.4 buffer, the dissolution kinetics were comparable for a P/Ca/P-system and Ca/P-system, the situation changes in a slightly acidic buffer at pH 6.0. In a phosphate buffer at pH 6.0 the dissolution of a P/Ca/P-system was faster than of a Ca/P-system due to the almost complete ionization of pectin at pH 6.0.
Summary
This case study highlights the controlled release profile of Metoprolol Tartrate as a water-soluble model drug. The formulation is based on CELLETS® 700, which serve as inert MCC spheres. By a variation in the multi-layer composition of calcium and pectin, the dissolution kinetics and controlled release profiles were examined.
Acknowledgement
This research was funded by Thailand Research Fund through Royal Golden Jubilee Ph.D. Program, grant number PHD/00005/2541.
References
[1] P. Wanasawas, A. Mitrevej, N. Sinchaipanid, Pharmaceutics 14 (2022) 1061, https://doi.org/10.3390/pharmaceutics14051061
[2] The American Society of Health-System Pharmacists. Archived from the original on 12 March 2014. Retrieved 21 April 2014. https://web.archive.org/web/20140312062359/http://www.drugs.com/monograph/metoprolol-succinate.html
[3] M. Khotimchenko, Int. J. Biol. Macromol. 158 (2020) 1110-1124. https://doi.org/10.1016/j.ijbiomac.2020.05.002
Abstract
This case study on Atomoxetine HCl pellets is a short abstract of the publication by Y.D. Priya et al. [1].
Atomoxetine is a medication used to treat attention deficit hyperactivity disorder (ADHD) [2]. The API is marketed under the trade names Atomoxetine, Atomoxe, Agakalin, and Strattera (initially launched) [3]. Atomoxetine is an extremely bitter API. As being initially launched for children as capsules or tablets, the paediatric compliance by improved taste-masking and the simplified administration to paediatrics are in focus of this study.
A multi-unit particulate pellet coating (MUPS) was selected as oral dosage form. The fluidized bed technology (with Wurster column) was employed for coating and layering processes. This is a well-known technology, which Is for instance offered by Glatt. Starter cores were coated with the API, followed by layering with a polymeric coating for which realized the taste-masking.
Atomoxetine layering
Starter cores are made of Microcrystalline Cellulose (MCC) in sizes comparable to CELLETS® 200, while a fair efficiency of drug layering was observed with the combination of HPMC (Hydroxypropyl methyl cellulose) and HPC (Hydroxypropyl cellulose) as binders. The composition of API layering is presented in Table 1. The drug dispersion was sprayed onto the MCC pellets with an inlet temperature between 50 °C and 55 °C and a fluidized bed temperature between 35 °C and 40 °C.
| API layering material | Composition |
| Starter core | |
| MCC pellets | 58.00 |
| API layering | |
| Atomoxetine HCl | 25.00 |
| Hydroxypropyl methylcellulose | 3.50 |
| Hydroxypropyl Cellulose | 3.50 |
| Low-Substituted Hydroxypropyl Cellulose | 5.00 |
| Talc | 5.00 |
| Purified Water | Qs |
| Total weight (mg) | 100.00 |
Table 1: Formulation of API layered pellets.
Taste-masking coating
The polymeric taste-masking layer is made of a methacrylate co-polymer (Eudragit EPO) providing an excellent coating with taste masking properties for fine particles and tablets. The composition of the taste-masking suspension is shown in Table 2. The inlet temperature is between 40 °C and 45 °C, and fluidized bed temperature is between 25 °C and 30 °C.
| Polymeric coating material | Composition |
| Drug Layered pellets | 100.00 |
| Eudragit EPO | 25.00 |
| Sodium Lauryl Sulfate | 2.500 |
| Stearic acid | 3.750 |
| Talc | 6.25 |
| FD&C Yellow No. 6 | 0.50 |
| FD&C Red No. 3 | 0.05 |
| Purified Water | Qs |
| Total weight (mg) | 138.050 |
Table 2: Formulation of polymeric coating suspension.
The efficiency of taste-masking was benchmarked by a bitterness rating on human volunteers. Figure 1 shows, that the taste sensitivity identifies a bitterness at 6 µg/ml API concentration and an extreme bitterness at 7 µg/ml API and higher concentration. Thus, the threshold bitterness of Atomoxetine HCl is 6 µg/ml.
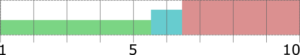
Figure 1: Concentration of drug solution (µg/ ml). Bitter intensity ratings from no bitterness (green), bitterness (blue), extremely bitter (red).
All the volunteers felt bitter taste when the drug layered pellets were coated with 6.25 mg of Eudragit EPO. Whereas in the pellets coated with 12.5 mg and 18.75 mg of Eudragit EPO, bitter taste was masked up to 15 seconds after keeping the tablet in the mouth, and later all the human volunteers felt bitter taste. When the concentration of Eudragit EPO was increased to 25 mg, the bitter taste of Atomoxetine HCl was completely taste-masked and no volunteer was felt bitter taste.

Figure 2: In-Vivo Taste evaluation in healthy human volunteers.
Figure 3 depicts the entire particle size of a taste-masked MCC pellet coated with the Atomoxetine drug layer and 25 mg of Eudragit EPO. The average particle size of the taste-masked pellets is between 180 µm and 250 µm, assuming, that no gritty feeling of particles in patient’s mouth will appear. It should be said, that a micronization of Atomoxetine HCl was deemed to be necessary for the drug layering process. Micronization minimized the surface roughness of the API layered pellet so that an efficient taste-masking coating can be applied.
Figure 3: SEM picture of cross section of a Taste masked pellets coated with 25 mg Eudragit EPO.
Summary
MCC pellets in the size of about 200 µm were layered with Atomoxetine. HPMC and HPC were used as binders, realizing a precise surface definition for a subsequent taste-masking coating. The taste-masking was most efficient at a polymeric concentration of 25 mg. Keeping the size of the coated pellets below 300 µm avoids a gritty feeling and thus increase the patient’s compliance.
This study by Priya et al. indicated that the fluidized bed process produced the most appropriate taste masked pellets of Atomoxetine HCl for oral disintegrating tablets.
References
[1] Y.D. Priya et al., Int J Pharm Pharm Sci, (6) 7, (2014) 110-115
[2] “Atomoxetine Hydrochloride Monograph for Professionals”. Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 4 April 2019. Retrieved 22 March 2019.
[3] ROTE LISTE 2017, Verlag Rote Liste Service GmbH, Frankfurt am Main, ISBN 978-3-946057-10-9, (2017) 162.

 ingredientpharm
ingredientpharm ingredientpharm
ingredientpharm

 ingredient pharm
ingredient pharm ingredientpharm
ingredientpharm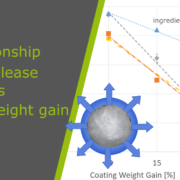 ingredientpharm
ingredientpharm