Case Studies

Cellulose-Derived Spherical Activated Carbon

Characterization of Layered Pellets and the Role of Amorphized Amlodipine Besylate / Hydrochlorothiazide

Cellulose-derived spherical activated carbon – sustainable nanoarchitectonics for efficient uremic toxin removal in pharmaceutical applications

Multiple-Unit Pellet System with Diclofenac Sodium

Scientific Literature on MCC Pellets: Insights into CELLETS®

Multiparticulate Oral Dosage Form of Tapentadol
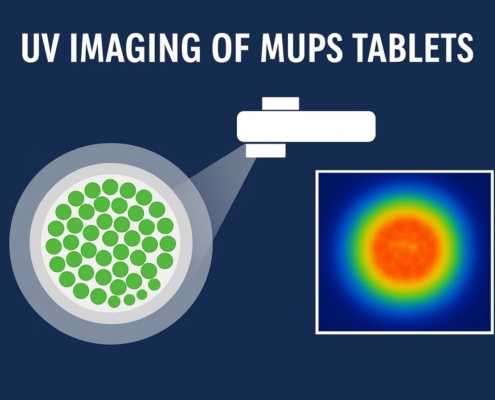
UV Imaging of MUPS Tablets: Stability, Functionality, and Outcomes

CELLETS® in Malodor Control Compositions: A Patent Overview

Hot-Melt Coating Materials: Enhancing Pharmaceutical and Industrial Applications
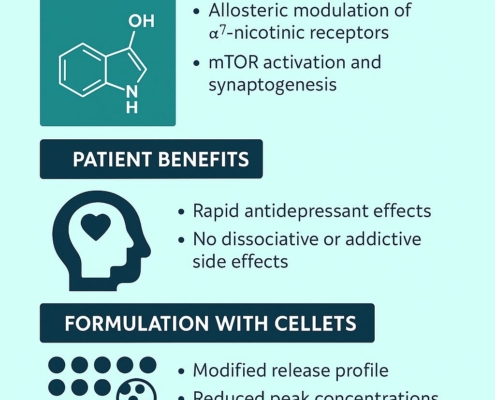
Patent on hydroxynorketamine modified‑release dosage form for the use in the treatment of depression

Patent on methods of administering gamma-hydroxybutyrate compositions with divalproex sodium

A regenerable microporous adsorbent based on microcrystalline cellulose for organic pollutants adsorption

Cellulose CELLETS as new type of adsorbent for the removal of dyes from aqueous media

Renewable Resource Biosorbents: Granulated Cellulose CELLETS 200 for Organic Pollutants Adsorption in Fixed-Bed Column Systems

Fixed-bed-column studies for methylene blue removal by CELLETS

Ultrasound Imaging of Artificial Tongues – an approach with Cellets
 https://cellets.com/wp-content/uploads/2025/05/fribility-ChatGPT-Image-14.-Mai-2025-14_26_12.jpg
1024
1024
Bastian Arlt
https://cellets.com/wp-content/uploads/2016/10/Logo_Cellets_2016_website.png
Bastian Arlt2025-05-16 15:38:022025-05-16 15:39:35Friability of MCC Pellets: importance in pharmaceutical formulations
https://cellets.com/wp-content/uploads/2025/05/fribility-ChatGPT-Image-14.-Mai-2025-14_26_12.jpg
1024
1024
Bastian Arlt
https://cellets.com/wp-content/uploads/2016/10/Logo_Cellets_2016_website.png
Bastian Arlt2025-05-16 15:38:022025-05-16 15:39:35Friability of MCC Pellets: importance in pharmaceutical formulations
Narrow and wide size distributions: Importance for MCC pellets in sustained-release oral dosage forms

Patent on solid oral dosage form comprising antibodies for sustained release in the lower gastrointestinal tract

Real-time monitoring of multiparticulate coating processes at industrial-scale using ultra-high-resolution optical coherence tomography
Patent on oral formulations of a Pyridinone derivate
 Adobe Firefly
Adobe FireflyPatent on enteric-coated particles containing lactoferrin

Revealing the physical restrictions of caecal influx in broilers through the use of solid and soluble markers
 adobe firefly
adobe fireflyConclusion of the Blog Series: Innovations in Controlled Drug Release

Patent on pharmaceutical compositions and methods for treating hyperhidrosis

Patent on gamma-hydroxybutyrate compositions having improved pharmacokinetics in the fed state

What does AI say about CELLETS?

A nutritional table for CELLETS®

In vitro validation of colon delivery of vitamin B2 through a food grade multi-unit particle system
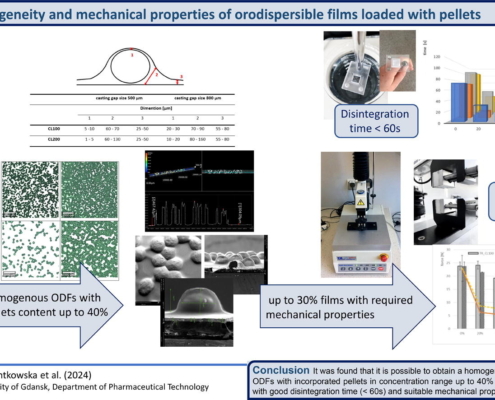
Homogeneity and mechanical properties of orodispersible films loaded with pellets
 adobe firefly
adobe fireflyPatent on packaged modified release gamma-hydroxybutyrate formulations having improved stability
 adobe firefly
adobe fireflyPatent on pulsatile release caffeine formulation
 adobe firefly
adobe fireflyPatent on modified-release Gamma-Hydroxybutyrate formulations having improved pharmacokinetics
 adobe firefly
adobe fireflyPatent on controlled release formulations of highly lipophilic physiologically active substances
 adobe firefly
adobe fireflyPatent on methods of administering gamma-hydroxybutyrate compositions with divalproex sodium
 adobe firefly
adobe fireflyPatent on modified release gamma-hydroxybutyrate formulations having improved pharmacokinetics

Patent on extended release compositions comprising pyridostigmine

Patent on Extended-release compositions comprising atomoxetine

Influence of Polymer Film Thickness on Drug Release from Fluidized Bed Coated Pellets and Intended Process and Product Control
 https://cellets.com/wp-content/uploads/2024/09/Firefly-How-to-improve-the-dissolution-performance-of-rivaroxaban.-18547-example-image-scaled.jpg
1463
2560
Bastian Arlt
https://cellets.com/wp-content/uploads/2016/10/Logo_Cellets_2016_website.png
Bastian Arlt2024-09-04 16:22:122024-10-17 18:10:22How to improve the dissolution performance of rivaroxaban?
https://cellets.com/wp-content/uploads/2024/09/Firefly-How-to-improve-the-dissolution-performance-of-rivaroxaban.-18547-example-image-scaled.jpg
1463
2560
Bastian Arlt
https://cellets.com/wp-content/uploads/2016/10/Logo_Cellets_2016_website.png
Bastian Arlt2024-09-04 16:22:122024-10-17 18:10:22How to improve the dissolution performance of rivaroxaban?
The Increase in the Plasticity of Microcrystalline Cellulose Spheres’ When Loaded with a Plasticizer
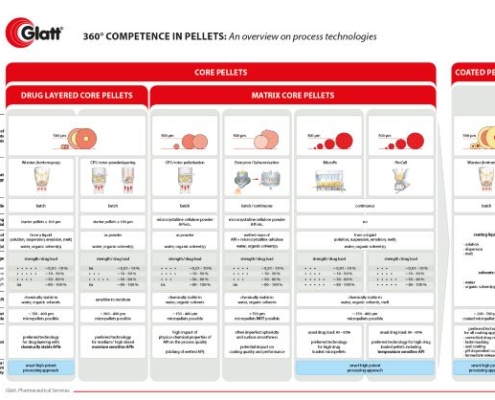 Glatt
https://cellets.com/wp-content/uploads/2021/01/pellets_overview_technologies_small.jpg
418
596
Bastian Arlt
https://cellets.com/wp-content/uploads/2016/10/Logo_Cellets_2016_website.png
Bastian Arlt2024-06-28 14:11:202024-10-15 19:01:50Download center
Glatt
https://cellets.com/wp-content/uploads/2021/01/pellets_overview_technologies_small.jpg
418
596
Bastian Arlt
https://cellets.com/wp-content/uploads/2016/10/Logo_Cellets_2016_website.png
Bastian Arlt2024-06-28 14:11:202024-10-15 19:01:50Download center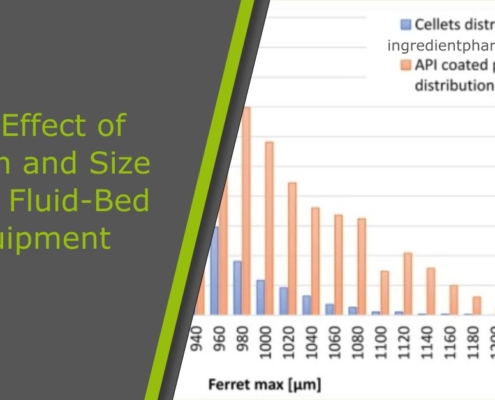
The Effect of Design and Size of the Fluid-Bed Equipment on the Particle Size-Dependent Trend of Particle Coating Thickness and Drug Prolonged-Release Profile
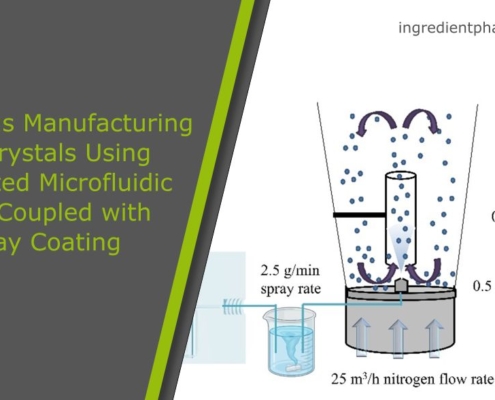
Continuous Manufacturing of Cocrystals Using 3D-Printed Microfluidic Chips Coupled with Spray Coating

Modelling the disintegration of pharmaceutical tablets: integrating a single particle swelling model with the discrete element method
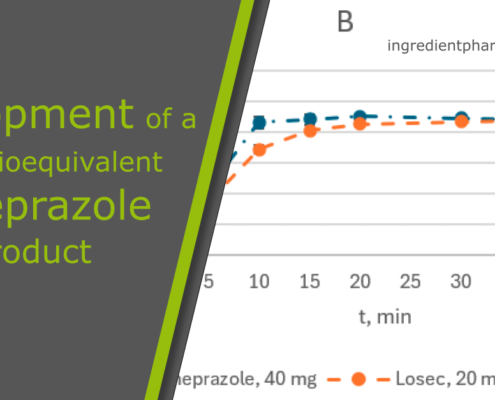 ingredientpharm
ingredientpharmDevelopment of a New Bioequivalent Omeprazole Product
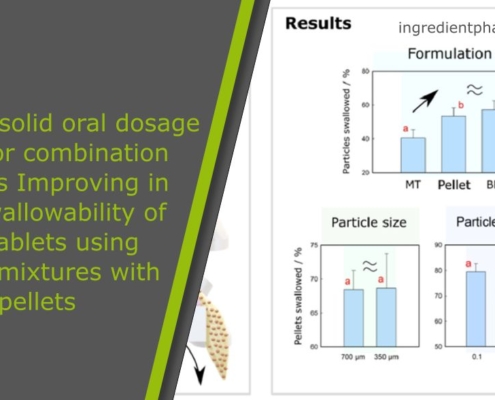
Paediatric solid oral dosage forms for combination products: Improving in vitro swallowability of minitablets using binary mixtures with pellets
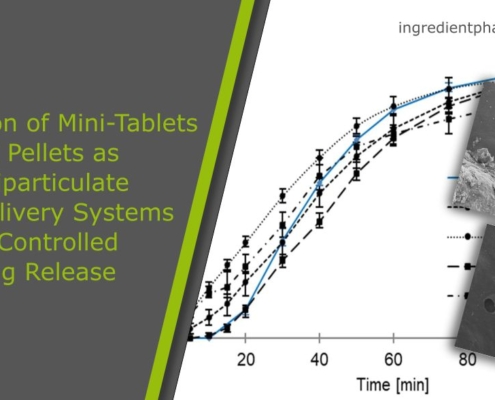
Comparison of Mini-Tablets and Pellets as Multiparticulate Drug Delivery Systems for Controlled Drug Release

Investigating the Influence of Fluid-Bed Equipment Design and Size on Particle Coating Thickness Trends and Drug Prolonged-Release Profiles Linked to Particle Size
 ingredientpharm
ingredientpharmCritical aspects of starter spheres in oral pellet formulations one should consider
 ingredientpharm
ingredientpharmPreparation and characterization of controlled-release doxazosin mesylate pellets using a simple drug layering-aquacoating technique
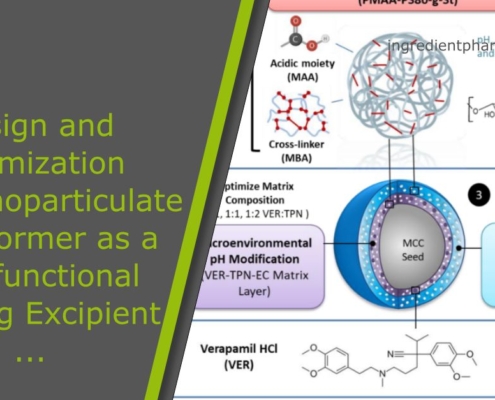
Design and Optimization of a Nanoparticulate Pore Former as a Multifunctional Coating Excipient for pH Transition-Independent Controlled Release of Weakly Basic Drugs for Oral Drug Delivery
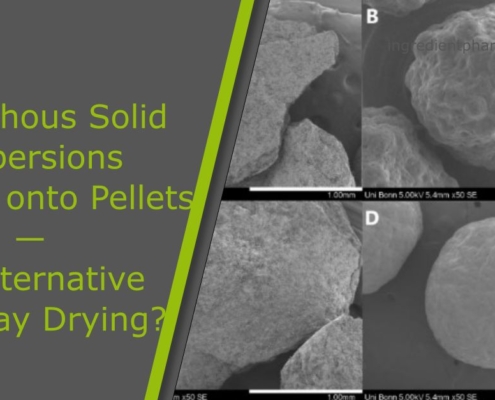
Amorphous Solid Dispersions Layered onto Pellets – An Alternative to Spray Drying?
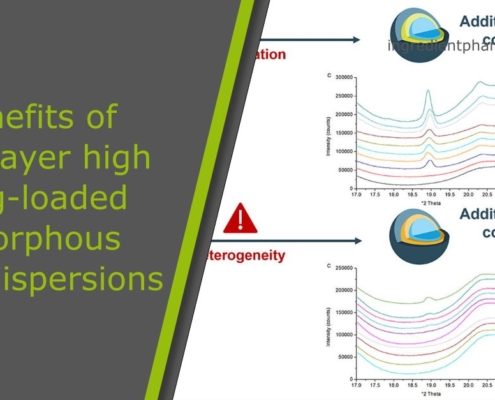
Benefits and challenges of multilayer high drug-loaded amorphous solid dispersions
 ingredient pharm
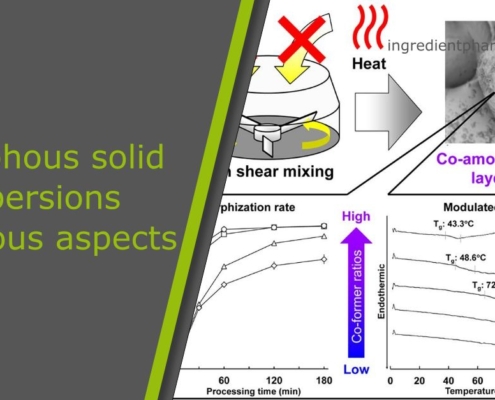
ingredient pharmAmorphous solid dispersions at various aspects
 ingredientpharm
ingredientpharmHydrodynamic behavior of MCC Spheres in a spouted bed
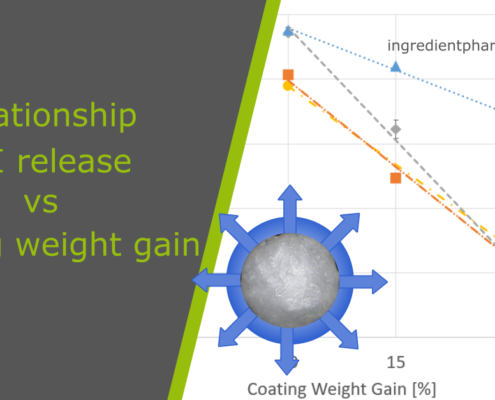 ingredientpharm
ingredientpharmCoating weight gain and API release in multiparticulates
Metoprolol Tartrate: controlled release drug layered pellets for colon-specific drug delivery
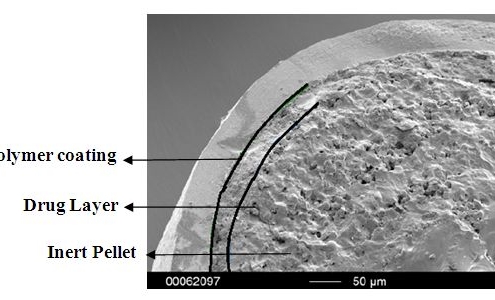
Atomoxetine HCl Pellets in MUPS Formulations

Tamoxifen citrate was spray layered micropellets for Cre recombination research
 ingredientpharm
ingredientpharmBSC Class I APIs in oral formulations
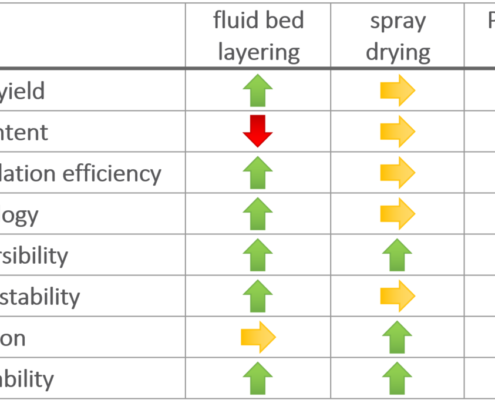 Ingredient Pharm
Ingredient PharmTechnologies for enhancing bioavailability of Simvastatin
Multidimensional Correlation of Surface Smoothness and Process Conditions

Gliclazide formulations for Sustained Release Delivery
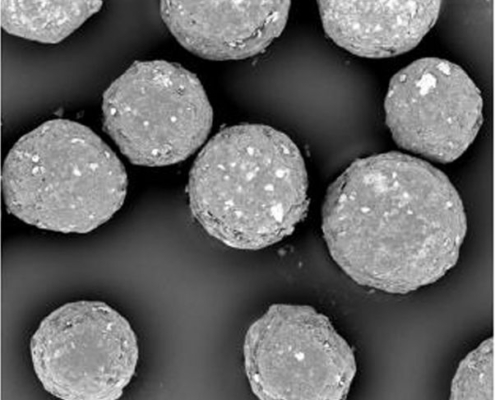
Microparticle coating in Wurster Fluidized Bed – Scalable Production of Oral Sustained-Release Drugs
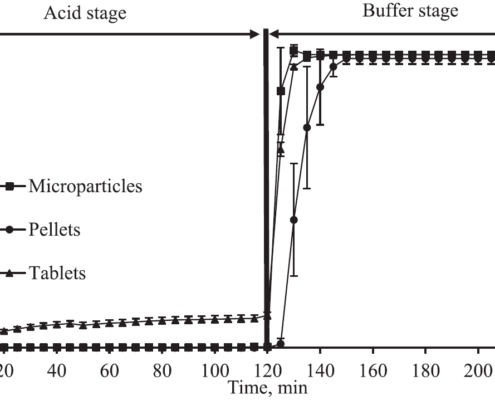
Faster drug release of enteric coated microparticles in bicarbonate buffers media
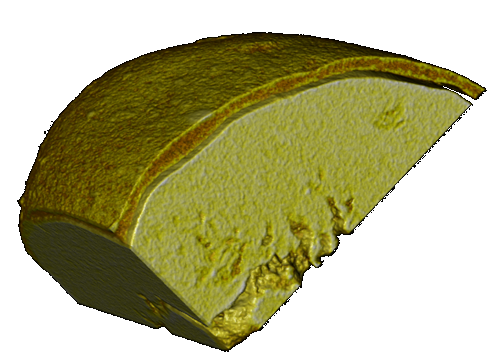
Coating uniformity of hot-melt coated particles
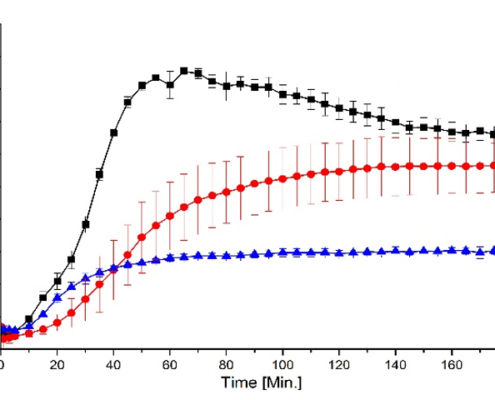
Amorphous solid dispersions layered pellets
 Ingredientpharm
IngredientpharmImpact of the particle size on powder behavior in a Wurster fluid-bed process
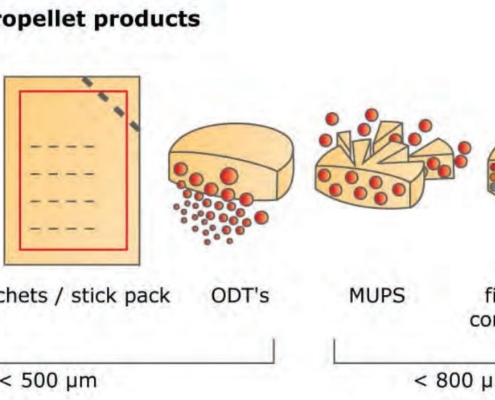 glatt.com
glatt.comThe path to the perfect sphere
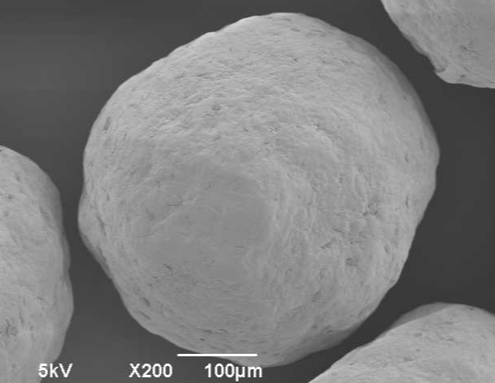 ingredientpharm
ingredientpharmSome light on sugar and microcrystalline cellulose pellets
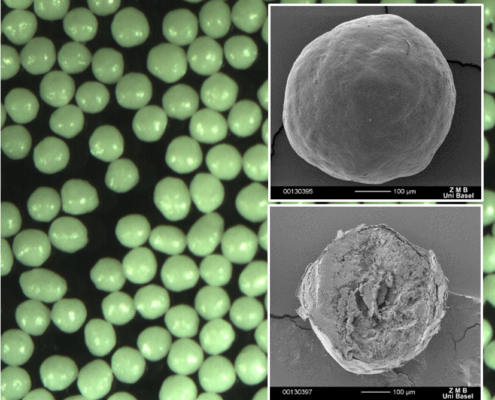 https://cellets.com/wp-content/uploads/2021/03/CS_hydrocortisone_image_5.png
699
827
Bastian Arlt
https://cellets.com/wp-content/uploads/2016/10/Logo_Cellets_2016_website.png
Bastian Arlt2021-03-04 13:01:512023-07-06 14:55:59Case Study: Hydrocortisone for paediatrics
https://cellets.com/wp-content/uploads/2021/03/CS_hydrocortisone_image_5.png
699
827
Bastian Arlt
https://cellets.com/wp-content/uploads/2016/10/Logo_Cellets_2016_website.png
Bastian Arlt2021-03-04 13:01:512023-07-06 14:55:59Case Study: Hydrocortisone for paediatrics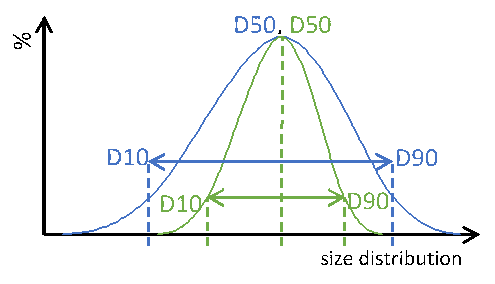
Sphericity and size distribution of µm-sized microcrystalline pellets
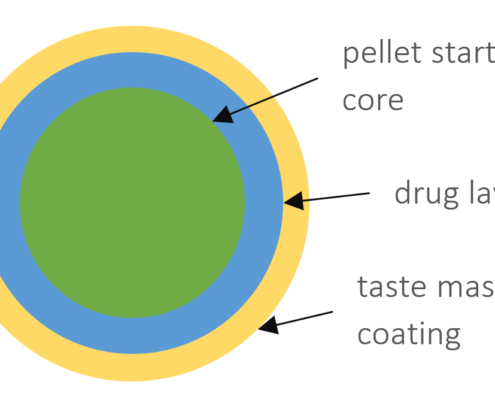
Taste-masked cellulose based pellets for compaction

Better MUPS tablets: Aspects of MCC starter beads

Case Study: Layering of Theophylline
Introduction to CELLETS® case studies
CELLETS® case studies show how microcrystalline cellulose spheres support pharmaceutical research, nutrition, and drug delivery. These studies highlight the versatility of CELLETS® as starter cores for controlled and modified release. They also demonstrate their role as food-grade carriers and solid markers in biological studies. Therefore, by reviewing CELLETS® case studies, it becomes clear how these particles advance drug targeting, improve pharmaco-kinetics, and enable innovation in both human and animal health.
A list of highly-rated scientific publications
MCC starter beads are frequently used in scientific publications. Main research is established in pharmaceutical applications, but we also identify CELLETS® in non-pharma research activities, such as in nutra, food and cosmetics. Impressively, these pellets help supporting young researchers in publishing their first poster, paper, publication in renowned journals and conferences. See the publication list here.
Recent applications of CELLETS® in pharmaceutical innovations
CELLETS® case studies reveal several pharmaceutical breakthroughs. Patents on pyridinone derivatives and enteric-coated lactoferrin use CELLETS® to deliver active compounds directly to the small intestine. In addition, they shield sensitive ingredients from stomach acid. Research on hyperhidrosis and gamma-hydroxybutyrate (GHB) also shows how CELLETS® enable modified-release systems. Moreover, they improve pharmacokinetics in fed conditions. See more here.
CELLETS® in nutrition and gut health research
CELLETS® case studies also explore nutrition and gut health. A food-grade delivery system with vitamin B2 uses CELLETS® as the foundation for colon-targeted release. As a result, nearly all of the vitamin is released exactly where it benefits gut function most. In addition, CELLETS® offer a safe, stable base for multi-unit particle systems that enhance nutritional therapies. See more here.
Scientific validation and practical insights from CELLETS® case studies
CELLETS® case studies provide strong scientific validation. For instance, in vitro tests confirm their effectiveness in targeted release. Furthermore, research in broilers uses CELLETS® as solid markers to track caecal influx. Comprehensive literature reviews also reinforce their credibility. In addition, nutritional tables and AI-based assessments show CELLETS® are widely accepted as reliable excipients. Consequently, these case studies demonstrate their broad value in pharmaceutical technology and nutrition science. See more here.
